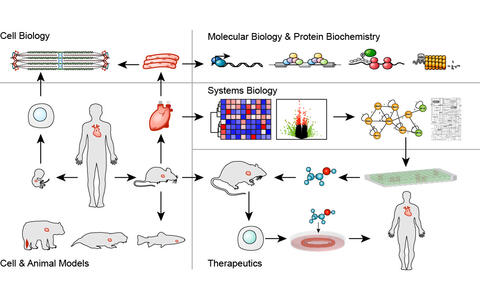
Gotthardt Lab
Translational Cardiology and Functional Genomics
Profile
Specifically, we are interested in understanding how alternative splicing relates to diastolic heart failure, how increased filling of the cardiac ventricle leads to improved contraction (Frank-Starling mechanism of the heart), and how the electrical properties of the heart and mechanoelectrical coupling are regulated.
The group focuses on titin, the largest protein in the human body, and the multifunctional coxsackie- and adenovirus receptor (CAR). To lay the groundwork for the in vivo analysis of titin's multiple signaling, elastic, and adaptor domains, the group has generated various titin knock-in and conditional knockout mice and established a tissue culture system to study titin's striated muscle functions and sarcomere dynamics.
We utilize a combination of cell-biological, biochemical, genetic, pharmacological, and omics tools to establish titin as a mechanosensor and therapeutic target. With a comparable loss of function approach we have created a conditional knockout of the coxsackie- and adenovirus receptor to demonstrate that CAR is crucial for embryonic development and determines the electrical properties of the heart. We currently develop CAR inhibitors to affect cardiac remodeling and improve cell based therapies.
Currently our main translational focus is on harnessing alternative splicing to improve cardiac function using a pharmacological screen with custom splice reporter assays. This work aims to identify and characterize patients with splice related heart disease towards improved personalized diagnostics and therapy.
Team
Research
A theory is something nobody believes, except the person who made it. An experiment is something everybody believes, except the person who made it.
Towards understanding how molecular machines such as the sarcomere are built and how biomechanics and cellular signaling relate, we focus on titin, the largest protein in the human body and the multifunctional coxsackie-adenovirus receptor (CAR).
To lay the groundwork for the in vivo analysis of titin’s multiple signaling, elastic, and adaptor domains, we have generated various titin deficient mice (knock-in and conditional knockout animals) and established a tissue culture system to study titin’s muscle and non-muscle functions. We utilize a combination of cell-biological, biochemical, and genetic tools to establish titin as a stretch sensor converting mechanical into biochemical signals.
Using a comparable loss of function approach we have created a conditional knockout of the coxsackie-adenovirus receptor. With these mice, we have demonstrated that CAR is crucial for embryonic development and determines the electrical properties of the heart.
- Cardiac Splicing
- Michael Radke, Vita Dauksaite, Victor Badillo Lisakowski, Jacobo López Carballo, Pragati Nalinkumar Parakkat
Alternative splicing is a process that determines which exons are included in the mature RNA and accounts for the majority of transcriptomic diversity. It is tightly regulated to accommodate the needs of the developing and aging organism in a changing environment. Only recently has the role of alternative splicing in disease been appreciated. While defects in splice factors (trans-effects) are rare, mutations in splice sites within disease genes (cis-effects) account for up to 30% of inherited disease.
Our goal is to investigate the concerted regulation of cardiac alternative splicing in development and disease and to evaluate if splice directed therapy can be used to improve diastolic function and specifically the elastic properties of the heart.
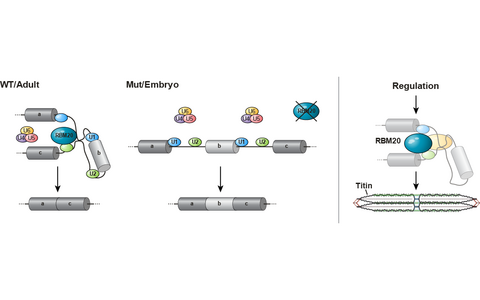
Regulation of cardiac function via alternative splicing.
RBM20 is a cardiac splice factor that promotes exon exclusion in the Wildtype (WT). Mutations (Mut) results in increased retention of alternative exons (left). RBM20 determines the elastic properties of the sarcomeric protein titin (right).- Titin Based Mechanotransduction
- Michael Radke, René Jüttner, Judith Hüttemeister, Pragati Nalinkumar Parakkat
Titin is a unique molecule that contains elastic spring elements and a kinase domain, as well as multiple phosphorylation sites. Therefore, it has been frequently speculated that titin and invertebrate giant titin-like molecules could act as a stretch sensor in muscle. More recently, this concept has been supported by studies on human dilative cardiomyopathies which suggest an impaired interaction of titin with its regulatory ligands Tcap/telethonin and MLP protein. However, so far it has remained unknown how the stretch signal is processed, i.e. how the mechanical stimulus stretch is converted into a biochemical signal.
To investigate the stretch signaling pathway, we apply mechanical strain in vivo (plaster cast for skeletal muscle; aortic banding for the heart) and in tissue culture (cultivation of primary cells on elastic membranes). The resulting changes in protein expression and localization in our titin kinase and spring element deficient animals are used to map the mechanotransduction pathway.
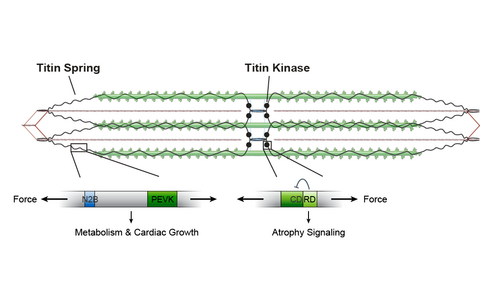
Titin based mechanotransduction.
The sarcomeric protein titin (black) contains a spring region and a kinase domain. Application of force leads to extension of the titin filament- Striated Muscle Development and Sarcomere Dynamics
- Michael Radke, Judith Hüttemeister
Overlapping titin molecules form a continuous filament along the muscle fiber in heart and skeletal muscle. Together with the multiple binding sites for sarcomeric proteins, this makes titin a suitable blueprint for sarcomere assembly. The use of transgenic techniques does not only allow us to address the function of titin’s individual domains in sarcomere assembly, but also to follow sarcomere assembly and disassembly using fluorescently tagged proteins. Understanding the structural and biomechanical functions of titin will help elucidate the pathomechanisms of various cardiovascular diseases and ultimately aid the development of suitable therapeutic strategies.
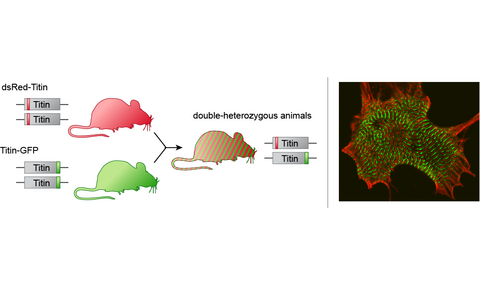
Visualizing sarcomere dynamics.
Generation of titin knock-in mice expressing RFP-Titin and Titin-GFP fusion proteins (left). Titin fusion proteins are properly integrated into the sarcomere (right). They produce the characteristic periodic Z-disc (red) and M-band staining (green).- The Coxsackie-Adenovirus Receptor in Health and Disease
- Rene Jüttner, Felizia Voß, Jacobo López Carballo
CAR was cloned as a receptor used by adeno- and coxsackievirus to enter cells but its physiological role has remained obscure. Detailed information on the expression pattern such as upregulation surrounding myocardial infarction and a critical role in embryonic development (lethality in mid-gestation of the CAR knockout) are well established, but no information on its role in the adult heart has been available.
We have generated both tissue culture and animal models to study CAR’s function in cardiac remodeling, inflammatory cardiomyopathy, and basic cellular processes such as endocytosis and cell-cell contact formation.
Our preliminary data suggest a critical role of CAR in the conduction of electrical signals from the atria to the cardiac ventricle. The inducible heart-specific knockout of CAR has enabled us to completely block the entry of coxsackievirus into cardiomyocytes and prevent all signs of inflammatory cardiomyopathy.
CAR is a multifunctional protein.
CAR (light green) is involved in cell contact formation, actin filament assembly, ion homeostasis (gap junctions), endocytosis, and several signal transduction pathways. It interacts with multiple extra- and intracellular proteins.