Proteomics and Metabolomics
Stefan Kempa
Profil
Interactions between genes, proteins, and metabolism are a key part of cellular processes. It is now possible to study the manifold molecular species and correlations in the cell at a systematic and quantitative level through large-scale analytical techniques such as mass spectrometry. Whereas proteomics (a term coined on the analogy of “genomics”) aims at the large-scale study of proteins and their functional networks, metabolomics is concerned with the intermediates and products of metabolism. Investigating the intertwined molecular systems allows for a better and more complex understanding of metabolic regulation and its impact on basic processes such as cell differentiation and cancer formation.
Cellular crosstalk in health and disease
The integrative proteomics and metabolomics research and technology platform is an integral part of the Berlin Institute for Medical Systems Biology (BIMSB). We are a team of biologists, engineers, chemists, pharmacologists, and bioinformaticians who have established a number of mass spectrometry-based proteomics and metabolomics methods that allow us to analyze the dynamics of metabolism under in vitro conditions and in living organisms.
For proteomics studies, we apply mass-spectrometric techniques that either use stable isotopes for labeling proteins or that are label-free. We have also developed and patented a specific workflow called pulsed stable isotope-resolved metabolomics (pSIRM) that provides a dynamic, time-resolved quantitative measurements of the central metabolism in in vitro and in vivo investigations.
Specifically, our group is interested in the crosstalk between metabolism and gene regulation during cellular differentiation and cancer formation. We are focusing on central metabolism, a set of core biochemical pathways that is highly flexible and continuously adjusted to the physiological programming – and reprogramming – of the cell. Inversely, the metabolic state and metabolic modifications can influence how, for instance, potentially cancer-causing genes (oncogenes) are expressed.
Pinpointing the role of metabolic stress
Moving forward, we will intensify our efforts to explore this crosstalk between metabolism and gene regulation. For example, the protein MYC is an important regulator of gene expression that, if mutated, can promote tumor growth. We are deciphering, on a molecular level, how the production of MYC is itself controlled by metabolic stress. We also aim to further improve our methods and data integration workflows to allow us to apply proteomics and metabolomics techniques to interactions at the single-cell level in healthy and in cancerous tissues.
Our tools
- Metabolomics.
- Stable isotope-resolved metabolomics
- Proteomics
Team
Technology & Applications
Cellular crosstalk in health and disease
The integrative proteomics and metabolomics research and technology platform is an integral part of the Berlin Institute for Medical Systems Biology. We are a team of biologists, engineers, chemists, pharmacologists, and bio-informaticians. In order to analyze the dynamics of metabolism under in vitro conditions and in living organisms, we have established a number of mass spectrometry-based proteomics and metabolomics methods.
For proteomics studies, we apply mass-spectrometric techniques that either use stable isotopes for labelling proteins or are label-free. Furthermore, we developed and patented a specific workflow (pulsed stable isotope resolved metabolomics, pSIRM) that provides a dynamic, time-resolved quantitative measurement of the central metabolism for in vitro and in vivo investigations.
Specifically, our group is interested in the crosstalk between metabolism and gene regulation during cellular differentiation and cancer formation. We are focusing on central metabolism, a set of core biochemical pathways, which is highly flexible and continuously adjusted to the physiological program – and reprogramming – of the cell. Inversely, the metabolic state and metabolic modifications can influence how, for instance, potentially cancer-causing genes (oncogenes) are expressed.
Using these techniques we are able to measure the metabolic activity of cells and organs in a dynamic manner in vitro and in vivo. With these techniques we are able to quantify proteins and metabolites from cells, organs and organisms and to monitor their turnover rates in a genome-wide scale.
Overview about the different mass spectrometers installed in our group and the related applications: (1) two dimensional gas chromatography coupled mass spectrometer [GCxGC-TOF); (2) nano LC / UPLC liquid chromatography coupled triple quadrupole mass spectrometer [LC-QQQ]; (3) nano LC liquid chromatography coupled LTQ-Orbitrap mass spectrometer <em>Software tools
- Software tools
PTXQC
Is a software tool that generates comprehensive quality reports from proteomics analyses. As input the analysis results of the MaxQuant software tool is used (Bielow et. al. under review).
MetMax
Is a software tool developed to generate data matrices from GC-MS based metabolomics analyses. It extracts areas or mass intensities from exported mass spectra for stable isotope resolved metabolomics analyses as input a CSV export of the chromatof-software is used. MetMax is constantly maintained by our group.
link: http://www.ncbi.nlm.nih.gov/pubmed/19206143
Maui-VIA
Is a software tool that enables a fast and precise analysis of GC-EI-MS metabolomics data. The software-tool allows the automated analysis of our in house developed identification (IDENT) and quantification (QUANT) mixtures and includes additional measures for quality control. Early in 2015 we have organized the 1st GC-MS and MAUI-SILVIA user workshop incl. members of BIMSB and MDC groups. The software tool is declared as invention at the MDC.
- Metabolomics and proteomics methods
The different mass spectrometric platforms that are established within our group allow the quantitative measurement of a broad spectrum of biomolecules - this setup offers a high flexibility for the establishment and the further development of metabolomics and proteomics techniques.
The analysis of metabolites and proteins is crucial to determine the metabolic network of cells and organs. Although transcript levels contain basic information about the metabolic settings, transcriptional studies will never be sufficient to describe the metabolic state. Because the regulation of metabolism appears at all biological layers: (post-) transcriptional, (post-) translational and on an allosteric level.
Our group established cutting edge mass spectrometry-based metabolomics and proteomics techniques to analyze cell cultures, organs and whole organisms. Proteomic techniques combined with stable isotope labelling as SILAC (stable isotope labelling of amino acids in cell culture) are used for quantitative proteome analyses. Gas chromatography coupled mass spectrometry (GC-MS) as well as liquid chromatography coupled mass spectrometry (LC-MS) based techniques are applied to monitor the metabolome. In addition, we have developed pulsed stable isotope resolved metabolomics (pSIRM) as a tool for a dynamic metabolic characterization of cellular metabolism. We use both strategies (I) direct infusion parent ion scan and neutral loss scan methods and (II) direct infusion high resolution applications for lipidome analyses.
Metabolic profiling / fatty acid profiling
Metabolic profiling (free fatty acid profiling) is an untargeted GC-MS based method that allows the quantitative analysis of a broad spectrum of small molecules. Using commercial and in house data analysis tools large data sets can be established as basis for downstream statistical and bioinformatics analyses.
pSIRM (pulsed stable isotope resolved metabolomics)
pSIRM is an in house established workflow that enables a quantitative and time resolved analysis of cellular metabolism. We have further developed the analysis of stable isotope incorporation in vivo (mouse models) and we have established methods that allow to decode the action of metabolic inhibitors (see below). In frame of a collaboration with Katharina Nöh from the Helmholtz center Jülich we are translating pSIRM data as input for metabolic modeling and calculation of metabolic fluxes. This work is performed in frame of the DynaMeTox project. Beside the publication of the workflow we have filed a patent to protect the intellectual properties of our inventions.
Lipid analyses (Lipidomics)
Lipids are an important class of metabolites that are crucial as structural components of biological membranes, signaling molecules and energy source. Lipids comprise the largest number among all classes of metabolites. In order to comprehensively analyze the lipidome of biological materials powerful methods are required. We use triple-quadrupol based parent ion and neutral loss scan methods as well as high resolution techniques to quantitatively measure the lipidome. A software tool for comprehensive data analysis is under development in context of the BIH metabolomics core.
Nucleotide analysis
Nucleotides and nucleosides are an important class of molecules that are building blocks of nucleic acids, signaling molecules and energy equivalents. Of special interest is the composition analysis of DNA and RNA and to quantify modified nucleotide species. We use triple-quadrupol SRM based methods to determine the composition of RNA; novel mass spectrometry based high resolution methods are in preparation. The new established methods will allow to measure nucelotides/nucleosides in an unbiased way.
Proteomics
Various proteomics methods were established in our lab. Using the established methods we have conducted a large number of projects and collaboration during the last years.
Veröffentlichungen
Nachrichten
Outlook
Pinpointing the role of metabolic stress
Moving forward, we will intensify our efforts to explore this crosstalk of metabolism and gene regulation. For example, the protein MYC is an important regulator of gene expression that, if mutated, can promote tumor growth. We are deciphering on a molecular level how the production of MYC is itself controlled by metabolic stress. In addition, we will further improve our methods and data integration workflows to allow applying proteomics and metabolomics techniques to interactions at single cell level within healthy and cancerous tissues.
Research Projects
Using the established mass spectrometry based metabolomics and proteomics methods we investigate metabolic dys-regulation in cancer and muscular dystrophies. Recently we build up a metabolomics platform for the Berlin Institute of Health aiming to develop metabolomics towards a tool for systems medicine and personalized medicine.
- Metabolic reprogramming
Metabolic reprogramming occurs during differentiation of stem cells, immune cell activation and also during oncogenic transformation. In the next years we envision to use the established proteomics and metabolomics tools to decode the molecular basis and function of such reprogramming events. During stem cell differentiation a shift of the cellular metabolism occurs. These events include the activation of mitochondrial metabolism detectable by increasing flux through TCA cycle intermediates. Interestingly, the state of the mitochondrial metabolism is very different between stem cells and cancer despite the fact that both cell types depend on glycolytic metabolism and show high rates of lactic acid production in cell culture (Warburg effect).
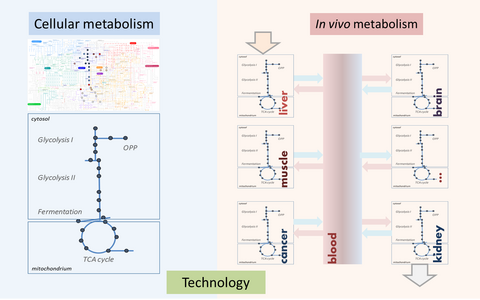
Illustration of the analytical strategies (I) investigating the cellular metabolism as a defined metabolic network and (II) analyzing the metabolic composition of blood that reflects the metabolic interplay of the different organs and the metabolic state in health and disease.
- Metabolic alterations during stem cell differentiation
By comparing the glycolytic activity of stem cells and cancer cells similar glucose derived fluxes can be measured. However, the steady state levels of glycolytic intermediates are quite different. Also the proteomic profiles between various cancer cell lines and stem cell differ at the isoenzyme level. We conclude that even though the Warburg effect can be observed in both conditions the underlying molecular machinery may be distinct. We aim to analyze the metabolic machines of stem cells and cancer cells and to correlate the results with metabolomics data to understand the difference between stem cell and cancer cell metabolism. Ultimately, we aim to understand if cancer cell metabolism can be targeted without disturbing proliferating cells in a healthy organism.
- Analysis of cancer cell metabolism
Cancer cells display a high metabolic activity allowing them to grow in a competitive manner inside the body. Those cells take advantage over neighboring cells by metabolizing faster the available resources, mainly glucose, glutamine and oxygen. An up-regulation of glycolysis and the resulting lactate formation of many tumors was already observed and described nearly 90 years ago by the Nobel laureate Otto Heinrich Warburg. Warburg termed this effect ‘aerobic glycolysis’ because he observed lactate formation, a product of an anaerobic metabolism, by tumor cells even in the presence of oxygen. The increased metabolic rates of cancer cells induce very fast a gradient of nutrient availability and produce an environment where glucose and oxygen become limiting. It is expectable that the cellular signaling and metabolic networks adjust to these conditions. Furthermore, we propose that cells respond differently to therapy upon altered environmental conditions even if the underlying genome is the same. We have started to characterize cancer cell metabolism in regard to nutrient availability. We often observed that metabolic activities are not necessarily reflected in the abundance of proteins or even in the steady-state levels of metabolites. Beyond being regulated simply by the expression levels of enzymes, the cellular metabolic pattern is influenced also by the isoform composition of the enzymatic network and its regulatory properties. Our lab participates in the e:Bio projects 'Hepatomasys' and 'Oncopath' aiming to decode the molecular interaction between the cellular signaling network and metabolism.
Model of the metabolic heterogeneity of solid tumors. The scheme illustrates the different zones of solid tumors classified by nutrient availability
We speculate that further metabolic pathways support proliferation of cancer cells. We and others found that nearly no glucose-derived carbon entered mitochondria and that glutamine was the carbon source for the tricarboxylic acid cycle (TCA-cycle). Further, we have evidence that this metabolic activity depends on oxygen levels. However, till now there is no clear evidence that cancer cells display such high glutamine consumption in vivo!