Rehm Lab
Translationale Tumorimmunologie
Deregulated Transcription Factors
Deregulated transcription factor networks and their role in dedifferentiation, reprogramming, proliferation and survival of malignant lymphomas
Cellular differentiation of hematopoietic stem cells into distinct lineages is controlled by a complex network of transcription factors that establish lineage-specific gene expression and/or suppress alternative developmental fates. During the last two decades, it has become increasingly clear that disruption of the physiological differentiation process is intimately linked to lineage infidelity, cellular reprogramming and eventually tumor development in the hematopoietic system.
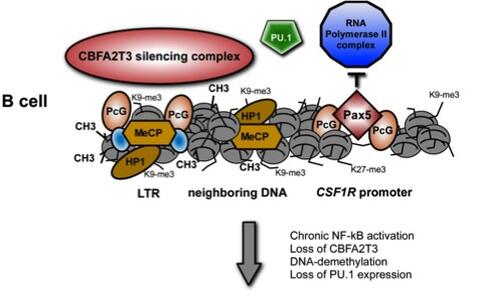
CSF1R promoter regulation in normal B cells and malignant Hodgkin Lymphoma tumor cells (HRS)
A number of lymphoid tumors display a phenotype that is in accordance with such a reprogramming process, including classical Hodgkin lymphoma (cHL), primary effusion lymphoma (PEL) and anaplastic large cell lymphoma (ALCL). Among these, cHL constitutes the most prominent example for lineage infidelity and reprogramming: in striking contrast to their origin from B-cells, the malignant Hodgkin-/Reed-Sternberg (HRS) cells of cHL have almost completely lost their B-cellspecific gene expression program and acquired expression of genes characteristic for other hematopoietic lineages. We have demonstrated that the B cell-associated transcription factor E2A is functionally inhibited in HRS cells by the upregulation of the antagonistic helix-loophelix proteins ID2 and ABF1, leading to a complete inhibition of the normal B cell-associated E2A DNA binding activity, suppression of B cell-specific genes and activation of non-B cell, i. e. lineage-inappropriate genes, among them CSF1R, GATA3 and TCF1.
Interestingly, we have observed in further studies that disruption of the physiological E2A activity is not limited to cHL, but can also be found in other lymphoma entities such as PEL, ALCL and T cell-derived Sézary syndrome, demonstrating that alterations in E2A activity belong to the most frequent recurrent functional aberrations in lymphoid neoplasms. As a striking example for lineage-inappropriate gene expression, we have identified the overexpression of the myeloid CSF1 receptor in B cell-derived HL, a phenomenon that mediates strong mitogenic and survival signals for HL cells. Remarkably, we observed that expression of the CSF1 receptor is not mediated by the canonical myeloid promoter, but by an upstream long terminal repeat (LTR) that is aberrantly activated due to a loss of epigenetic control.
(S.Mathas, M.Janz)