Izsvák Lab
Mobile DNA
The impact of Transposable Elements (TE)-derived activities on disease mechanisms
Project #2
Transposable elements contribute to the evolution of human placentation, and might explain some human-specific features of pathological pregnancies
Katarina Stevanovic, Julia Rugor*, Rabia Anwar*, Yuliang Qu*
In placenta, in comparison to other tissues, the genomic noise, generated by various TEs is relatively high. In collaboration with Prof. R. Dechend (Charité) and Prof. L. D. Hurst (University of Bath, UK) our aim is to investigate epigenetic regulation and imprinting abnormalities in the pathogenesis of preeclampsia (PE), a pregnancy disorder associated with cardiovascular diseases. We are aiming at discovering TE-affected gene regulation, TE-derived transcripts, and their dysregulation in PE. Our ultimate goal is to find diagnostic molecules that are released from the placenta into the blood stream, and could be established as biomarkers of PE.
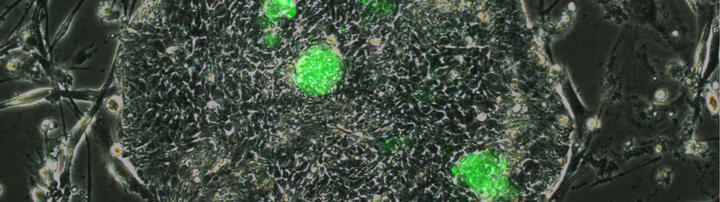
LINE-1 activity in human trophoblast
Katarina Stevanovic
Preeclampsia (PE) is a leading pregnancy disorder, yet its etiology remains unknown. The heterogeneous and complex features of the disease might be associated with disturbed epigenetics, as indicated by the dysregulation of transposable elements (TEs) and imprinted genes (ie. DLX5). Transcriptome analyses of patient-derived samples revealed that mutagenic LINE-1 retrotransposons are abundantly expressed in the trophoblasts of the placenta, when compared to other tissues, and are upregulated in certain PE patient-derived samples. The overall goal of this project is to understand the role (if any) of LINE-1 transcription (transposition?) in placenta development and their potential contribution to PE pathogenesis.
Dysregulated trophoblast-specific gene expression mediated by retroviral regulatory sequences contributes to preeclampsia (PE)
Rabia Anwar*
The current study was inspired by the following hypothesis. Transposable element-(TE)-derived sequences are frequently co-opted in placental gene regulation. As TE-derived sequences are sensitive to epigenetic disturbances, their upregulated transcription might dysregulate placental gene expression, and thus contribute to the placental pathogenesis (e.g. preeclampsia (PE)). Therefore, first we focused on identifying human endogenous retroviral LTR-derived elements that drive trophoblast-specific gene expression. We followed our assumption that trophoblast-specific genes that are regulated by LTR-derived elements might have an important role in human placentation and their dysregulation might lead to pregnancy related disorders such as PE. As each species has its own specific set of ERVs (), we further hypothesized that ERV-derived placental gene regulation might explain - at least - some human specific features of PE development. This strategy identified already known, but also new candidate genes contributing to PE pathogenesis. From the candidate list 6 genes were both confirmed to be driven by LTR-derived enhancers, and found to be significantly dysregulated in early-onset PE women. To delve deeper into the functional analysis of the novel candidate genes in PE pathogenesis, we focused on EPS8L1. Importantly, dysregulated expression of EPS8L1 is also detectable in the serum of PE women of a high-risk pregnancy cohort, with a potential of establishing it as a new biomarker of PE.
Human endogenous retrovirus K in glioblastoma
Dr. Christine Römer*, Islam Karimov, Manvendra Singh*
TLR8 is a human-specific intracellular receptor that recognizes single-stranded RNA, among others the human endogenous retrovirus K (HERV-K). We hypothesize that endogenously released endogenous retrovirus HERV-K functions as a ligand for the TLR8 in glioblastoma tumor tissue and aggravates tumor progression.
This work is in a collaboration with Prof. Dr. Seija Lehnardt, Charite
Human Endogenous Retrovirus K Rec forms a Regulatory Loop with MITF that Opposes the Progression of Melanoma to an Invasive Stage
Manvendra Singh* and Huiqiang Cai*
The HML2 subfamily of HERV-K (henceforth HERV-K) represents the most recently endogenized retrovirus in the human genome. While the products of certain HERV-K genomic copies are expressed in normal tissues, they are upregulated in several pathological conditions, including various tumors. It remains unclear whether HERV-K(HML2)-encoded products overexpressed in cancer contribute to disease progression or are merely by-products of tumorigenesis. Here, we focus on the regulatory activities of the Long Terminal Repeats (LTR5_Hs) of HERV-K and the potential role of the HERV-K-encoded Rec in melanoma. Our regulatory genomics analysis of LTR5_Hs loci indicates that Melanocyte Inducing Transcription Factor (MITF) (also known as binds to a canonical E-box motif (CA(C/T)GTG) within these elements in proliferative type of melanoma, and that depletion of MITF results in reduced HERV-K expression. In turn, experimentally depleting Rec in a proliferative melanoma cell line leads to lower mRNA levels of MITF and its predicted target genes. Furthermore, Rec knockdown leads to an upregulation of epithelial-to-mesenchymal associated genes and an enhanced invasion phenotype of proliferative melanoma cells. Together these results suggest the existence of a regulatory loop between MITF and Rec that may modulate the transition from proliferative to invasive stages of melanoma. Because HERV-K(HML2) elements are restricted to hominoid primates, these findings might explain certain species-specific features of melanoma progression and point to some limitations of animal models in melanoma studies.
- PMID: 33202765, PMCID: PMC7696977 DOI: 10.3390/v12111303


