Willnow Lab
Molecular Cardiovascular Research
Profile
We study genes associated with both metabolic disorders and Alzheimer’s disease, aiming to provide explanatory models for metabolic dysfunctions as major cause of neurodegeneration. We elucidate gene functions using humanized mouse models and validate the clinical relevance of our findings in studies involving patients and iPSC models thereof. In this reporting period, we focused on a class of intracellular sorting receptors, called VPS10P domain receptors, identifying essential roles for directed protein transport in control of cell viability and function common to metabolic and brain cell types. We uncovered molecular mechanisms whereby receptor (dys)functions cause rare familial but also common sporadic disorders of the metabolism and the ageing brain, paving the way for therapeutic strategies to combat age-related dementia - devastating conditions impacting millions of patients worldwide.
Team
Research
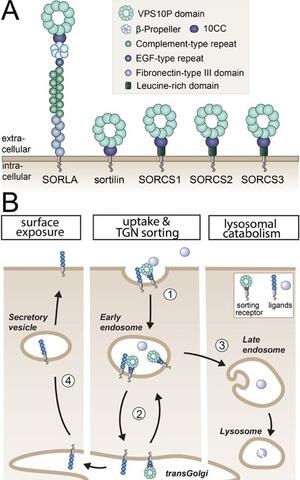
Fig. 1: Structure and sorting paths of VPS10P domain receptors (A) VPS10P domain receptors are mammalian type-1 transmembrane proteins characterized by a vacuolar protein sorting 10 protein (VPS10P) domain that constitutes the main ligand binding site. Their extracellular domains may carry additional motifs for protein interactions, while their cytoplasmic tails bind sorting adaptors that direct these receptors, and their bound cargo, to distinct intracellular compartments. SORLA, sorting protein-related receptor with class A repeats; SORCS, sortilin-related receptor CNS expressed. (B) Typically, VPS10P domain receptors internalize from the cell surface into early endosomes (step 1). From there, they shuttle between endosomal compartments and the transGolgi network (TGN), the central hub for intracellular protein sorting (step 2). Ligands internalized from the cell surface include soluble proteins directed to lysosomal catabolism (step 3). This pathway provides cells with essential metabolites and controls the extracellular concentration of signaling molecules. A second category of ligands are internalized co-receptors which are redirected via the TGN back to the cell surface, a mechanism defining the surface receptor repertoire in target cells (steps 1, 2, and 4) (Malik and Willnow, Trends Neurosci 2020).
Age-related neurodegeneration, as in Alzheimer disease (AD), is a major health burden in aging societies around the globe. Disturbingly, metabolic disorders, such as diabetes and obesity, emerge as major risk factors for AD, causally linking two health problems of epidemic proportions. While the interconnection between metabolic disturbances and neurodegeneration imposes considerable challenges for public health, they also offer hope that drugs proven their worth in treatment of metabolic disorders may be repurposed for diseases of the ageing brain.
It is our overall goal to elucidate molecular mechanisms linking metabolic disorders and neurodegeneration and to explore therapies for AD based on new or re-emerging drug targets.
Our research strategy focuses on functional elucidation of genes associated with both neurodegenerative and metabolic traits in humans as they may provide explanatory models for the co-morbidity of the ageing brain and systemic metabolism. A particular focus of our recent work has been a group of sorting receptors, called VPS10P domain receptors (Fig. 1A). Our earlier work uncovered their unique roles in protein trafficking in neurons (Jansen et al., Nat Neurosci 2007; Vaegter et al., Nat Neurosci 2011; Glerup et al., Mol Psych 2016) (Fig. 1B). Yet, receptor functions explaining their genetic association with neurodegenerative and metabolic disorders remained unresolved.
Recently, we elucidated novel disease mechanisms underlying the co-morbidity between the ageing brain and the metabolism. We identified the receptor SORLA as a key player in control of insulin signaling, impacting amyloid deposition in brain (in AD) and pancreas (in type 2 diabetes). We uncovered the central role of sortilin as neuronal apolipoprotein (apo) E receptor, essential to sustain a neuroprotective brain lipid metabolism. These later findings finally provide a molecular explanation for the risk of AD seen with the APOEε4 gene variant, the most important genetic risk factor for sporadic AD. Our studies on SORCS2 identified novel mechanisms in glial stress response protecting from acute or chronic insults to the brain. Our success in modeling congenital human brain disorders in iPSC-derived cell models laid the technological foundation for a major grant from the Novo Nordisk Foundation to explore the metabolic basis of AD.