Rathjen Lab
Developmental Neurobiology (Emeritus)
Profile
Moreover, we study neuronal mechanisms implicated in ultrasonic vocalizations and focus on several topics of the “wiring” problem. The correct wiring of the brain is of critical importance for its function. It is orchestrated by multiple molecular factors and cellular mechanisms during developmental periods.
Contact
Research
A neuron establishes up to thousands of synapses which are the fabric of the communication within the nervous system. The number, strength as well as specificity of synapses and the balance between inhibitory and excitatory neurons determine brain function. A longstanding goal of neuroscientists therefore is to understand how the enormous degree of connectivity of neurons is established during embryonic and early postnatal development and how this connectivity becomes modulated by experience-dependent processes. Dr Rathjen´s laboratory focuses on two subtopics of the “wiring” problem: on (1) the branching of axons and on (2) links between electric activity and proteins implicated in structural modeling of neuronal circuits.
Projects
- A cGMP signaling cascade composed of CNP, Npr2 and cGKI is implicated in the bifurcation of DRG axons
The branching of axons is a morphological hallmark of virtually all neurons, and allows an individual nerve cell to innervate several distinct targets and to communicate with neurons in different parts of the brain. Axonal branching is therefore a key mechanism in the generation of neuronal circuits and allows the processing of information arriving from different parts of the body. Thus, axonal branching is crucial for normal brain function and impairments of branching might result in neurological and neurodevelopmental disorders. In the last decades axonal branching has been therefore the subject of intense study primarily by in vitro cultures, but our knowledge of the underlying molecular signaling mechanisms is still fragmentary.
Many neurons within the brain display extremely complex patterns of axonal arborization. In contrast, projections of dorsal root ganglion neurons (DRG) into the spinal cord display a stereotyped and simple pattern of axonal branching. The central projections of DRG neurons branch at three sites in a temporally controlled order: DRG axons enter the spinal cord and bifurcate into two arms resembling a ‘T’ or ‘Y’ (first order branch), and then extend in rostral and caudal directions. Roughly two days later, the two daughter axons generate collaterals which grow in a ventral direction (second order branch). These collaterals terminate in distinct laminae of the spinal cord where they branch a third time (terminal branching). Due to this simple pattern of branching the spinal cord represents an attractive system to study factors implicated in axonal branching.
Our detailed in vivo analysis using transgenic techniques showed that a cGMP-dependent signaling cascade composed of the ligand CNP (type C natriuretic peptide), the receptor guanylyl cyclase Npr2 (natriuretic peptide receptor 2, also termed GC-B) and cGKI (cGMP-dependent kinase I, also termed PKGI) is essential for axonal branching at the dorsal entry zone of the spinal cord. In the absence of any one of these components, DRG axons do not bifurcate but are able to turn either rostrally or caudally (Figure 1). In contrast, collateral formation – second order branch - is not affected by this signaling system.
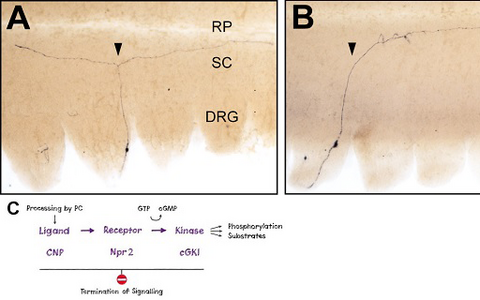
Figure 1: Loss of bifurcation of axons from DRG neurons in the absence of Npr2-mediated cGMP signaling.
Individual DRG neurons were labeled by a transgenic technique in wild-type mice (A) or in Npr2-mutant mice (B). The arrow heads point to a bifurcating and a non-bifurcating axon in the wild-type and Npr2-mutant mouse, respectively. Rostral is to the left. DRG, dorsal root ganglion; RP, roof plate; SC, spinal cord; scale bar, 250 µm. C) A cGMP-dependent signaling cascade composed of the ligand CNP (natriuretic peptide C), the receptor guanylyl cyclase Npr2 and the kinase cGKI (cGMP-dependent kinase I) is implicated in the branching of sensory axons when entering the spinal cord.- The cGMP signaling pathway that triggers bifurcation of DRG axons is also an essential regulator of the arborization of central afferents from cranial sensory ganglia
In the past years, we asked which neuronal populations apart from DRG neurons use this signaling system to regulate axonal branching. We observed that Npr2 and cGKI are strongly expressed in all sensory ganglia of the cranial nerves at embryonic stages coinciding with the entry of their central afferents into the brainstem. In addition, we detected a strong complementary expression of the ligand CNP in the alar plate of rhombomeres 2, 4, and 6/7 of the hindbrain, where trigeminal (V), facial (VII), vestibulocochlear (VIII), glossopharyngeal (IX) and vagal (X) sensory axons enter the hindbrain.
Using a transgenic technique to label selectively a subset of Npr2-positive neurons, we demonstrated that the absence of Npr2 causes a complete loss of bifurcation of all cranial sensory axons, which instead establish only either ascending or descending projections (Figure 2). Thus, axonal branching of cranial and dorsal root ganglia is regulated in an analogous manner by this cGMP signaling cascade. In contrast, collateral formation from the stem axons at the lateral margin of the hindbrain was not affected in the absence of cGMP – as also observed for DRG axons. In conclusion, a cGMP-dependent signaling pathway controls first-order bifurcation but not higher order branching of sensory neurons entering the CNS at any level (Figure 3).
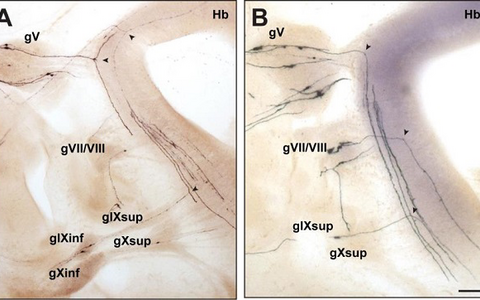
Figure 2: Bifurcation of axons from cranial sensory neurons depend on the receptor guanylyl cyclase Npr2.
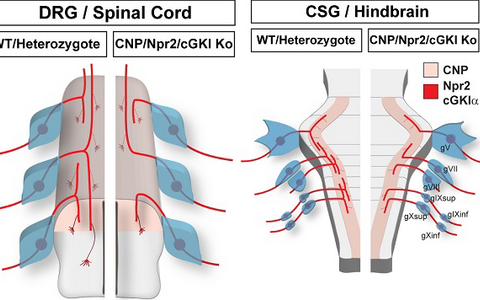
Individual neurons from cranial sensory ganglia were labeled by a transgenic technique in wildtype (A) or in Npr2-mutant mice (B). The arrow heads point to bifurcating and non-bifurcating axons in the wild-type and Npr2 mutant mouse. gV, trigeminal ganglion; gVII, geniculate ganglion; gVIII, vestibular and spiral ganglion; gIX, glossopharyngeal ganglion; gX, vagus ganglion; sup, superior; inf, inferior; Hb, hindbrain; scale bar, 200 µm.Figure 3: Schematic representation of the bifurcation defects observed in the absence of cGMP signaling in axons from dorsal root ganglia (DRG) in the spinal cord and cranial sensory ganglia (CSG) in the hindbrain. The localization of Npr2 and cGKI or CNP in the axons, spinal cord or hindbrain is colored. The left part illustrates the wild-type, the right the mutant situation. R1-r8, rhombomeres 1-8.
- Link between electric activity and cell adhesion molecules at developmental stages of the brain
In the mature brain electric activity is the language for communication between neurons. However, during embryonic and postnatal development electric activity of neurons is also implicated in shaping the structure of neuronal circuits of the brain. As soon as functional circuits forms, spontaneous activity in form of calcium waves becomes correlated between neighbouring neurons which contributes to the refinements of established circuits. Cell adhesion molecules (CAM) might be considered as candidates to link electric activity of neurons to structural changes within neuronal circuits. CAMs establish initial cell-cell contacts and provide a platform for contact mediated intercellular signaling. Four structural groups of CAMs are expressed in the developing nervous system: cadherins/protocadherin; IgCAMs (immunoglobulin cell adhesion molecules), integrins and neurexins/neuroligins. Of these several IgCAMs are expressed at early stages and reveal a strong localization on axonal surfaces. We have therefore asked whether IgCAMs influence electric activity itself during development and tested whether the absence of an IgCAM affects calcium signalling. We observed that the IgCAM CAR specifically modulates intracellular calcium levels by regulating internal calcium stores. Our studies revealed an unexpected link between adhesion processes, calcium release and electric activity.
Further characterization indicated that CAR is a typical cell adhesion molecule that is part of a specific subgroup of CAMs of the Iimmunoglobulin superfamily. CAR is primarily expressed in the developing nervous system and heart and is almost absent at mature stages. It reveals homophilic as well as heterophilic interactions. Our structural, binding as well as adhesion studies predict a flexible ectodomain of CAR allowing conformational shifts for cis or trans homophilic interactions on neurons.
- Impaired presynaptic function and elimination of synapses in the absence of CALEB
CALEB, also termed neuroglycan C or CSPG5, is a neural protein composed of an N-terminal segment that contains chondroitin sulfate chains followed by an acidic stretch, an EGF-like domain, a transmembrane and a cytoplasmic segment. CALEB expression is restricted to the central nervous system and appears to be generated as a precursor protein that becomes converted to a truncated transmembrane form with an exposed EGF domain and a secreted form. The conversion from a long form to a truncated form is promoted by electric activity. Our previous investigation on this protein showed an impaired synapse function in the absence of CALEB at developing but not mature stages.
In past years we studied the role of CALEB in the developing cerebellum which was motivated by our observation that CALEB-deficient mice displayed impaired motor coordination. Analysis of the neuronal connectivity of Purkinje cells by patch-clamp recordings demonstrated impairments of presynaptic maturation of inhibitory synapses. GABAergic synapses on Purkinje cells revealed decreased evoked amplitudes, altered paired-pulse facilitation and reduced depression after repetitive stimulation at early postnatal but not at mature stages.
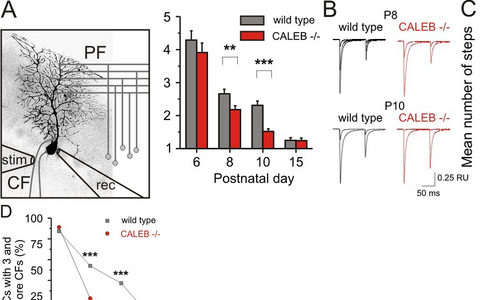
Furthermore, the elimination of supernumerary climbing fiber synapses on Purkinje cells was found to be impaired in the absence of CALEB. For example, at postnatal day 8 in wild-type mice 54 percent of Purkinje cells have three or more climbing fiber synapses in contrast to mutants where this number is decreased to less than 25 percent (Figure 4). The basic properties of the climbing fiber Purkinje cell synapse remained unaffected. The alterations observed by patch-clamp recordings correlated with a specific pattern and timing of expression of CALEB in Purkinje cells. It is dynamically regulated during development from a high chondroitinsulfate-containing form to a non-chondroitinsulfate-containing form. Thus, our results demonstrated an involvement of CALEB in presynaptic differentiation of cerebellar GABAergic synapses and revealed a role for CALEB in synapse elimination in Purkinje cells. These observations are restricted to early postnatal stages suggesting a transient role for CALEB in synapse maturation.
Figure 4: Elimination of climbing fiber-Purkinje cell synapses is impaired in the absence of CALEB.
The scheme (A) illustrates the electrophysiological design to estimate the number climbing fiber synapses on Purkinje cells. Example traces (B) and mean number of steps (C), representing the mean number of synapses from wild-type and CALEB -/- Purkinje cells. D) The percentage of Purkinje cells with three and more climbing fiber synapses at different developmental stages in the absence of CALEB in comparison to wild-type Purkinje cells is shown. CF - climbing fiber; PF - parallel fiber; rec - recoding pipette; stim - stimulation pipette.