Current DFG funding for MDC researchers
The starting gun has been fired for the new Collaborative Research Centres (CRCs) of the German Research Foundation (DFG). In addition, the DFG is for the first time supporting 33 research projects on SARS-CoV-2 infection as part of its COVID-19 Focus Funding initiative. This new funding instrument features a simplified application process that enables researchers to quickly address particularly urgent questions related to the coronavirus pandemic. Scientists from the Max Delbrück Center for Molecular Medicine in the Helmholtz Association (MDC) are involved in two new CRCs and one COVID-19 research project.
Healthy blood vessels – strong bones
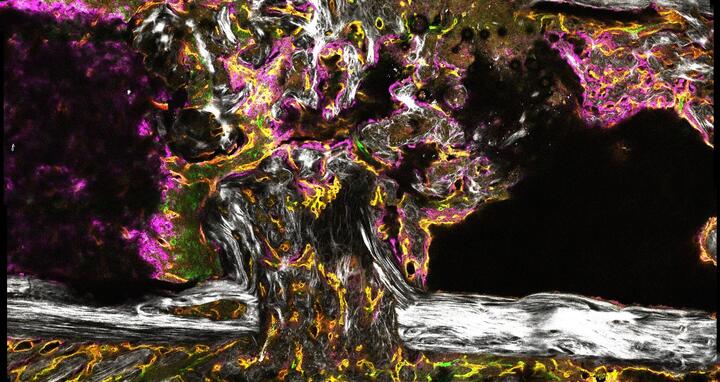
Angiogenesis in healing bone of a mouse, two weeks after osteotomy
The Collaborative Research Centre “Directed Cellular Self-Organisation for Advancing Bone Regeneration” (CRC 1444) aims to unravel the basic mechanisms that differentiate between success and failure in bone regeneration. Bone tissue is one of the few human tissues that heals without scarring. This makes bone an ideal model system for understanding general principles of endogenous healing and cellular self-organization. These processes basically work well in young and healthy people, but undergo changes caused by increasing age, a lack of exercise, chronic inflammation and metabolic disorders. This explains why musculoskeletal diseases and disorders occur more frequently in older people. A deeper understanding of how the body’s own healing processes change due to aging, metabolic disorders or altered immune responses – scientists call this “immunoaging” – is largely lacking. Yet such an understanding is required to enable the individualized treatment of these patients. Until now they have all basically received the same treatment, despite the fact that the healing potential of bone varies greatly in different patient groups.
CRC 1444 aims to contribute to this understanding. The DFG will initially fund the collaborative project for four years to the tune of over €12 million. Charité - Universitätsmedizin Berlin is the host university. Professor Holger Gerhardt’s Integrative Vascular Biology Lab at the MDC is involved in the project, as are scientists from the Berlin Institute of Health (BIH), Freie Universität Berlin, the Zuse Institute Berlin (ZIB), the Max Planck Institute of Colloids and Interfaces (MPIKG) and the German Institute of Human Nutrition (DIfE). “We’re interested in the human body’s vascular networks,” explains Gerhardt. Little is known about how new blood vessels form in healing bone. In the CRC, the MDC researchers are investigating the signaling pathways by which blood vessel cells in bone influence the healing process. In doing so, they are building on the work of the DFG Research Unit 2165 “Regeneration in Aged Individuals: Using bone healing as a model system to characterize regeneration under compromised conditions.”
Protective mucous barriers inside the body
What’s special about this consortium is that it brings together expertise in fundamental physical and biological processes in order to gain a complete understanding of the mechanisms that influence hydrogel formation.
MDC researchers are also involved in the Collaborative Research Centre “Dynamic Hydrogels at Biointerface” (CRC 1449). Hydrogels have an important barrier function: They cover the mucous membranes that line cavities in the body, such as the digestive and respiratory tract, protecting them from viruses and bacteria. Hydrogels consist of water-insoluble polymers, which can absorb large quantities of water and which swell up in the process. In diseases such as cystic fibrosis, chronic obstructive pulmonary disease, or some inflammatory bowel diseases, the mucous membranes are severely dehydrated. In the CRC, the scientists aim to determine the key physicochemical factors of hydrogels and investigate at the molecular level how they change and cause disease. In doing so, they intend to define the requirements for the development of new therapeutic strategies for pulmonary and gastrointestinal diseases.
“Targeted drugs could be developed to restore the protective function of the mucosa that has been destroyed by disease,” says Sofia Forslund, venturing a look into the future. She heads a research lab at the MDC that focuses on “Host-Microbiome Factors in Cardiovascular Disease.” Her work in the CRC explores how the microbiome influences hydrogel composition and can strengthen or destroy the mucous barrier. Also participating in the CRC project, along with Forslund, is Dr. Philipp Mertins, head of the MDC’s Proteomics Platform. He and his team want to advance the technology so that the proteins and glycoproteins in the hydrogels can be better characterized. To this end they are collaborating with the group led by Kevin Pagel, Professor of Organic Chemistry at Freie Universität Berlin.
“What’s special about this consortium is that it brings together expertise in fundamental physical and biological processes,” Forslund says, “in order to gain a complete understanding of the mechanisms that influence hydrogel formation.” The CRC has a duration of four years. Freie Universität Berlin is the host university, and the spokesperson is Professor Rainer Haag.
Uncovering how the coronavirus spreads
Coronaviruses have multiple ways of confusing the immune system (...) A deeper understanding of these mechanisms will help us to understand exactly what makes SARS-CoV-2 so contagious.
The COVID-19 projects supported by the DFG are funded for one year with €3.6 million each. Together with Michael Sattler and Grzegorz Popowicz, from Helmholtz Zentrum München – German Research Center for Environmental Health and Technische Universität München (TUM), Professor Markus Landthaler from the MDC and his research lab “RNA Biology and Posttranscriptional Regulation” are working on the project entitled “Biochemical and structural characterization of SARS-CoV-2 non-structural protein 16 (Nsp16), a cap ribose 2'O-methyltransferase.”
The SARS-CoV-2 virus has building instructions for only about two dozen proteins in its genome – in humans there are more than 20,000, which are also produced in a large number of variants. A few of these proteins act as scaffolding around which virus particles are formed. Others, and this includes Nsp16, ensure that the virus can multiply rapidly in human cells and hide from the immune system. Scientists suspect that Nsp16 alters the genetic material of the virus so that it is no longer recognized as “viral” in the cell. This, for example, may help the virus spread before people have symptoms. “Coronaviruses have multiple ways of confusing the immune system so that they can spread as quickly as possible,” says Landthaler. “A deeper understanding of these mechanisms will help us to understand exactly what makes SARS-CoV-2 so contagious.”
Text: Jana Ehrhardt-Joswig