How mitochondria stay in shape
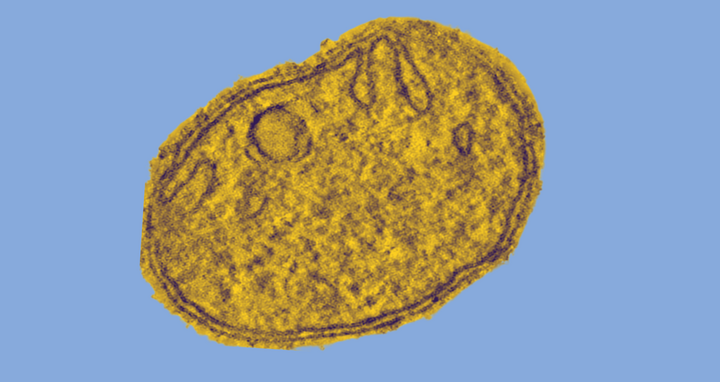
Mitochondria are the cellular powerhouses of nearly all organisms that have a cell nucleus. They produce the cell’s universal energy source: adenosine triphosphate (ATP). Depending on its energy needs, an individual cell can have up to several thousand mitochondria. An especially high number of these tiny organelles are found in nerve, muscle, and egg cells.
Mitochondria are believed to have evolved about two billion years ago from independent bacteria living in symbiosis with other bacteria. They have their own, circular genome and are surrounded by a double membrane. The outer membrane forms an elongated envelope. In contrast, the inner membrane is intricately folded and has numerous extended invaginations, called “cristae,” that resemble the fingers on a glove. ATP is produced at these hollow structures.
We have supplied the first molecular model for how crista junctions maintain their shape.
Until now, little was known about how the inner mitochondrial membrane maintains its characteristic shape, especially the highly curved neck regions of the cristae, the crista junctions. They are especially important, as they regulate the passage of matter in and out of the crista. “We have supplied the first molecular model for how crista junctions maintain their shape,” says Prof. Oliver Daumke, one of the paper’s two last authors and head of a lab at the Max Delbrück Center for Molecular Medicine in the Helmholtz Association (MDC). As researchers from Berlin, Hamburg, Göttingen, and Halle write in Science Advances, mitochondria are a prime example of how membrane form and function interact. Defective mitochondrial membranes can lead to neurodegenerative and muscular diseases. For example, defects in the Mic60 protein, which is instrumental in the formation of crista junctions, are connected with Parkinson’s disease.
A vault-like protein structure
“Using bacteria, we biosynthetically manufactured parts of the Mic60 and Mic19 proteins and then grew crystals from them, in order to visualize the structures in detail. At the BESSY II synchrotron in Berlin-Adlershof, the crystals were subsequently bombarded with X-rays to determine their 3D structure,” explains Dr. Tobias Bock-Bierbaum, who shares first authorship of the paper with Dr. Kathrin Funck.
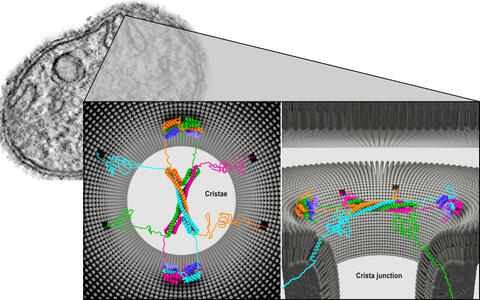
A protein complex of four Mic60 and four Mic19 proteins appears to be located at the neck of the crista. Constructed like the ribs of a Gothic vault, the complex may completely cover the crista junction. The Mic60 molecules are twisted together to form elongated helices.
Architecture of the Mic60-Mic19 complex at Crista junction (left: top view; right: side view).
Using bacteria, we biosynthetically manufactured parts of the Mic60 and Mic19 proteins and then grew crystals from them, in order to visualize the structures in detail.
Two pairs of proteins form a bow tie-shaped tetrameric assembly. The two ends are each flanked by two Mic19 proteins and are thus held together like a clasp. In addition, the protein vault is very dynamic. It can collapse and rebuild itself.
Daumke was surprised at how perfectly the proteins created in the lab assemble in the test tube. “We hypothesize that, perhaps depending on the size of the molecules, the entire complex regulates the passage of substances into and out of the crista junctions like a molecular sieve.” This structure would then be like a porous rubber cap atop a bottle’s neck.
This “molecular sieve” is so tiny that it cannot be clearly seen inside cells even with the best electron microscope. But there may be indirect evidence of its existence. “If the architecture of the protein complex – which so far we have only manufactured parts of in a test tube – really is crucial for stabilizing crista junctions, then the shape of the inner mitochondrial membrane would have to change when one of the proteins is defective,” Bock-Bierbaum explains.
In vivo evidence in yeast cells
The authors of the paper from left to right: Tobias Bock-Bierbaum, Kathrin Funck, Oliver Daumke
A research team led by Daumke’s partner in the study, Prof. Martin van der Laan at Saarland University in Homburg/Saar, tested this hypothesis on living cells by swapping out certain amino acids in the Mic60 protein of the yeast Saccharomyces cerevisiae. The result was clear: the crista junctions collapsed. “The cristae were still there, but they were sealed off between two smooth, round membrane layers,” said Bock-Bierbaum.
The researchers’ next goal is to examine the dynamics of the barrier more closely and to try to manufacture the entire protein structure. In addition, it is still unclear how the disease-causing structural defects come about. For example, according to Daumke there is a cellular regulator that is connected to Parkinson’s disease. “Now we want to study if and how it affects the architecture of the Mic60 protein complex.”
Text: Catarina Pietschmann
Further information
Literature
Bock-Bierbaum et al. (2022): „Structural insights into crista junction formation by the Mic60-Mic19 complex“. Science Advances, DOI: 10.1126/sciadv.abo4946.