Willnow Lab
Molecular Cardiovascular Research
Sortilin
A key player in neuroprotective lipid metabolism in the brain
Introduction
Sortilin is a 95 kDa receptor highly expressed in liver and brain. Previously, we uncovered its function as a lipoprotein receptor, controlling release of cholesterol-rich lipoproteins from hepatocytes and explaining its genetic association with hypercholesterolemia and risk of myocardial infarction (Kjølby et al., Cell Metab 2010). Interestingly, the encoding gene SORT1 also shows genome-wide association with AD and with frontotemporal dementia (FTD), the second most common form of age-related dementia. The underlying receptor function remained unknown. Because sortilin acts as receptor for uptake of apolipoprotein (apo) E-containing lipoproteins in neurons, we proposed a role in neuronal lipid homeostasis to explain its association with neurodegenerative diseases (Carlo et al., J Neurosci 2013). We substantiated this exciting hypothesis in this reporting period.
Sortilin is required for the neuroprotective action of apoE3
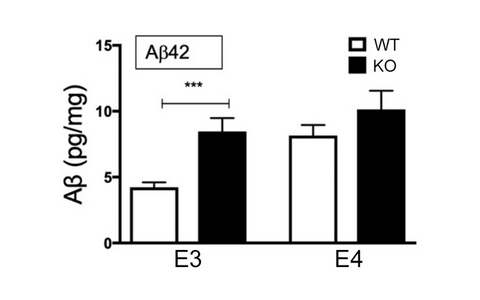
Fig. 4: Aβ levels in hippocampal extracts of mice expressing human apoE3 or apoE4 and being WT or deficient for Sort1 (KO). Values are mean ± SEM (n=8-12 per group). Significant differences between genotypes analyzed by Student’s t test
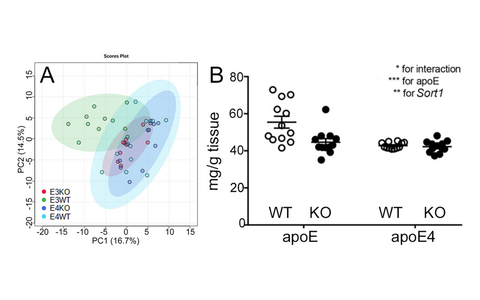
ApoE is the major lipid transporter in the brain. Secreted from astrocytes, it delivers essential lipids to neurons that take up apoE-bound cargo via apoE receptors, such as sortilin. ApoE is encoded by gene variants ε2, ε3, and ε4 in the human genome. ε4 is the most significant risk factor for sporadic AD, increasing disease prevalence in homozygous carriers 12-fold as compared to carriers of the common ε3 variant. To test whether sortilin function explains the risk of AD seen with apoE4, we generated humanized mouse models of the brain lipid metabolism, expressing either human apoE3 or apoE4 and being wildtype (WT) or deficient (KO) for Sort1. Aβ levels were low in E3WT mice but increased in E3KO animals (Fig. 4). By contrast, Aβ levels were always high in E4 mice, regardless of the presence (E4WT) or absence (E4KO) of sortilin. We concluded that sortilin is required for neuroprotective actions of apoE3 and that this activity is lost with apoE4. Differences in the brain metabolome of E3WT mice as compared to E3KO, E4WT, or E4KO animals argued for interaction of sortilin and apoE3 in brain metabolism to underly their neuroprotective function (Fig 5A). This model was substantiated by brain lipidomics, documenting levels of docosahexaenoic acid (DHA), the most abundant neuroprotective polyunsatu-rated fatty acid (PUFA), to be high in E3WT brains but pathologically low in all other genotypes (Fig. 5B).
We further validated our hypothesis, ultimately documenting that sortilin mediates neuronal uptake of PUFAs bound by apoE3. Internalized PUFA are metabolized to endocannabinoids (eCB), lipid-based neuromodulators that act as ligands for nuclear peroxisome proliferator-activated receptors (PPARs) to induce anti-inflammatory gene expression in neurons. Absence of sortilin in apoE3 mice (E3KO), or the presence of apoE4, disrupts this neuroprotective lipid metabolism and results in pro-inflammatory insults to the brain, accelerating Aβ production (Asaro et al., Alzheimer’s Dementia 2020).
Fig. 5: (A) Principal component analysis based on 550 metabolites quantified in cortical brain extracts of mice of the indicated genotypes (MS/MS-based Biocrates q500 assay; n=10 animals per group). (B) Levels of w-3 polyunsaturated fatty acid DHA as determined by LC-MS in brain cortices of mice of the indicated APOE and Sort1 genotypes (n=12 per group). Data are mean ± SEM. Significance of data was determined by two-way ANOVA.
ApoE4 disrupts the neuroprotective actions of sortilin
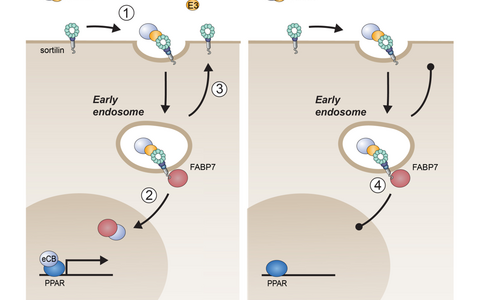
Fig. 6: Sortilin-dependent neuronal lipid homeostasis. (Left) Sortilin mediates uptake of PUFA bound by apoE3 (step 1). In endosomes, lipids are transferred to FABP7 bound to the sortilin tail. FABP7 directs further transport and metabolism of PUFA, ultimately delivering eCBs to nuclear PPARs to induce anti-inflammatory gene expression (step 2). Following lipid transfer, apoE3 and sortilin recycle to the cell surface to sustain neuronal lipid import (step 3). (Right) Because of the noxious tendency of apoE4 to aggregate in the acidic milieu of endosomes, internalized sortilin bound by apoE4 cannot recycle to the cell surface. Further uptake of PUFAs ceases and anti-inflammatory gene expression is lost (step 4). Thus, apoE4 renders neurons essentially deficient for sortilin activity, explaining why phenotypes in E4 mice are indistinguishable between Sort1 WT and KO genotypes.
Sortilin binds apoE3 and apoE4 with similar affinities (Carlo et al., J Neurosci 2014), leaving us with the puzzle why interaction with apoE4 would not support neuronal PUFA metabolism? We addressed this question by quantitative proteomics to identify hitherto unknown ligands dependent on sortilin for neuronal sorting. Our approach was based on the assumption that such ligands would exhibit altered subcellular localization in neurons lacking this sorting receptor. MS-based comparative surface proteomics uncovered several proteins aberrantly sorted to and from the cell surface in KO neurons, including fatty acid binding protein (FABP) 7, the neuronal transporter for PUFA and eCBs (Asaro et al., J Cell Sci 2021). Further studies uncovered the molecular concept explaining the detrimental impact of apoE4 on sortilin action in neuronal lipid handling (see Fig. 6 for details).