Scheidereit Lab
Signal Transduction in Tumor Cells (Emeritus)
NF-κB signaling in hair follicle organogenesis
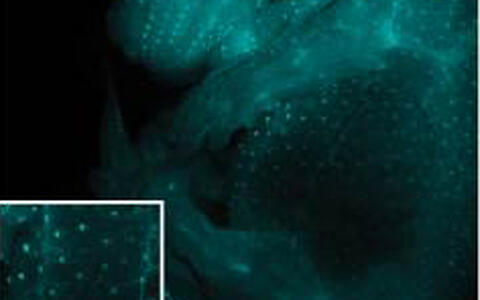
Fig. 1 EGFP expression under the control of NF-κB in developing hair follicles at E14.5
A potential role of NF-κB in organ morphogenesis remained obscure for long because of functional redundancy of NF-κB/Rel subunits and early embryonic lethality of relA, Ikkβ and Ikkγ knock-out mouse models. A knock-in mouse model, ubiquitously expressing an NF-κB super-repressor (IκBαΔN, ΔN) in the β-catenin locus (cIκBαΔN) unearthed a key role for NF-κB in the initiation of hair follicle development, and the morphogenesis of most other ectodermal organs, such as teeth and mammary glands, as well as of secondary lymph nodes (Schmidt-Ulrich et al., 2001).
Development of hair follicles requires an intense molecular interaction between the early embryonic ectoderm and the underlying mesenchyme (Schmidt-Ullrich & Paus, 2005). This leads to focal formation of an epithelial thickening (placode), which will grow into the mesenchyme and will eventually give rise to a hair follicle. Some essential signals which regulate early hair follicle development include WNT/β-Catenin, TNF family member ectodysplasin A1 (EDA-A1) and its receptor EDAR, SHH, and BMPs and their antagonists (Schmidt-Ullrich & Paus, 2005).
Fig. 2 Reciprocal interaction between WNT and EDA/EDAR/NF-κB signaling in early hair follicle development.
Using our ΔN-expressing and NF-κB reporter (κ-Gal and κ-EGFP, Fig.1) mice, as well as Eda-A1 and Edar loss-of-function mice, we have learned that epithelial EDA-A1/EDAR signaling specifically activates NF-κB in hair placodes (Schmidt-Ullrich et al., 2006). We were also able to show that epithelial WNT/β-Catenin signaling is absolutely required for NF-κB activation, and that Edar is a direct target gene of WNT/β-Catenin (Zhang et al., 2009). While WNTβ-catenin is mandatory for priming keratinocytes to adopt hair follicle fate, the downstream EDA-A1/EDAR/NF-κB signaling cassette controls refinement of placode patterning and possibly placode down-growth (Zhang et al., 2009) (Fig. 2). The integration of NF-κB signaling into WNT and SHH signaling networks may have important implications for the regulation of NF-κB in other physiological processes and in tumorigenesis. Detailed gene array analyses also revealed an important role for NF-κB in priming the developing hair follicle placode for subsequent down-growth into the underlying mesenchyme, a process similar to EMT.
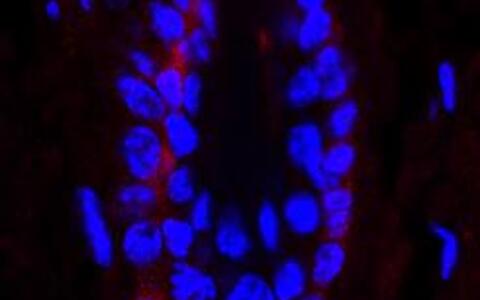
Fig. 3 NF-κB activity in the secondary hair germ (SGH) in a telogen hair follicle (in red).
The SGH contains activated hair follicle stem cells which eventually will give rise to a new hair.
A recently initiated project addresses the role of NF-κB in the hair cycle. Once the hair follicle has matured after birth it enters the first hair cycle, which will be repeated periodically throughout life. The hair follicle is the only organ in mammals that undergoes cyclic transformations, going from an organ growth phase (anagen) to apoptosis-driven regression (catagen), followed by a relative quiescent phase (telogen) (Schneider et al., 2009), which prepares the hair follicle for yet another anagen phase. Initiation of anagen phase involves hair stem cell activation and interaction of stem cells with the dermal signaling center of the hair follicle, the dermal papilla (Schneider et al., 2009). This interaction recapitulates molecular processes of embryonic hair follicle development and growth, strongly indicating that NF-κB might be involved in the hair cycle. First results suggest a function for NF-κB in anagen development and hair shaping (bending).
Researchers
- Karsten Krieger
- Ruth Schmidt-Ullrich
Collaborations
- Sarah Millar (Dept. of Dermatology and Cell & Developmental Biology, University of Pennsylvania, Philadelphia)
- Ralf Paus (University of Münster, Department of Dermatology, Münster, and University of Manchester, The Centre for Dermatology Research, Manchester).