Therapeutics Development
VONVENDI
VONVENDI is the only recombinant treatment for adults living with von Willebrand disease (VWD), an inherited bleeding disorder. Patients lack a protein – known as von Willebrand factor – that is crucial for normal blood clotting. VONVENDI provides a substitute and therefore helps control bleeding episodes.
Blincyto
BLINCYTO is an immunotherapy against an aggressive form of blood cancer (B-cell acute lymphoblastic leukemia). The bone marrow of these patients produces too many immature white blood cells. Rather than maturing into functional cells, these lymphoblasts rapidly reproduce, suppressing normal blood formation. The drug enlists the body’s own T-cells to destroy this cancer.
Current developments
Therapeutics in development by our industry partners
- Antibody targeted Amanitin Conjugates (ATAC)
-
-
HDP-101 is an Amanitin payload-based antibody drug conjugate (ATAC®) targeting BCMA for the treatment of multiple myeloma. The antibody of HDP-101 was developed at the MDC and licensed to Heidelberg Pharma (HDP). After preclinical testing, the development candidate has emerged as a clinical candidate.
HDP is a biopharmaceutical company in the field of oncology focusing on the development of antibody drug conjugates (ADCs) for the treatment of oncological diseases. The proprietary Amanitin payload-based ADCs uses Amanitin as the active ingredient. The biological mechanism of action of the amanitin toxin represents a new therapeutic principle.
ADC-Technologie – Heidelberg Pharma AG (heidelberg-pharma.com)
- Cellular immunotherapies to treat cancer and autoimmune disorders
-
-
The off-the-shelf CAR NK cell cancer immunotherapy FT576 is a first-of-kind, investigational treatment of multiple myeloma in development by Fate Therapeutics. This therapy incorporates four functional modifications including MDC’s proprietary B-cell Maturation Antigen (BCMA)-targeting CAR generated in the MDC research groups of Armin Rehm and Uta Höpken.
Fate Therapeutics is a clinical-stage biopharmaceutical company dedicated to bringing a first-in-class pipeline of induced pluripotent stem cell (iPSC)-derived cellular immunotherapies to patients with cancer and autoimmune disorders.
- CARTemis
-
CAR-T-cell immunotherapies for the treatment of cancer
-
The research groups led by Dr. Uta Höpken, Dr. Armin Rehm are working on cell-based immune therapies using so-called CAR T cells. These are specific immune cells taken from the patient that are genetically modified outside the body in such a way that they can recognize and destroy cancer cells. CAR T-cell therapy has already shown remarkable success in international studies for leukemias and lymphomas, but the MDC researchers are now developing novel CAR T cells that are particularly suitable for cancer of the hematopoietic system. The group has already generated the necessary CAR receptors in multiple variants. It also has a bioreactor that can produce the cell therapeutics in line with good manufacturing practice. The spinoff CARTemis will advance the commercial application of these MDC’s proprietary CAR-T cell therapies.
- Captain T-Cell
-
T-cell immunotherapies for the treatment of cancer
-
CAPTAIN T CELL develops an innovative approach to cancer immunotherapy. The goal is to create a platform for the production of personalized, cancer-specific T cell receptors. With the help of these receptors, the body’s own T cells, so called ‘killer cells’, are able to find and specifically destroy cancer cells in the patient’s body.
- TRYPTO THERAPEUTICS
-
treating serotonin-dependent diseases with novel Tryptophan hydroxylase 1 inhibitors
-
TRYPTO THERAPEUTICS is developing a set of novel, proprietary small molecules to treat serotonin-related diseases, such as Pulmonary Arterial Hypertension (PAH), Carcinoid Syndrome, Non-alcoholic steatohepatitis (NASH), and Fibrotic and inflammatory diseases. The spinoff team originated from the MDC research group of Prof. Michael Bader has developed and patented a novel class of TPH inhibitors (TPHi) that reduces pathologically increased peripheral serotonin with nanomolar potency in vitro.
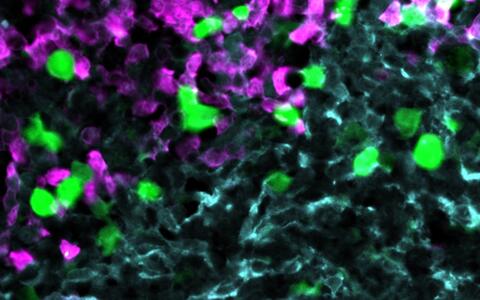
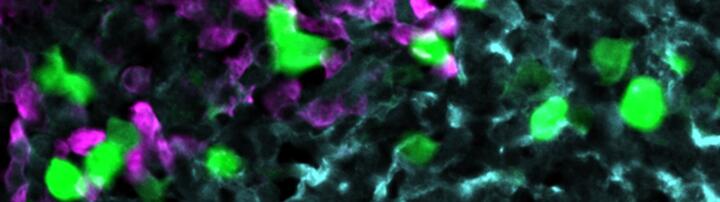
- About the header image
-
Novel CAR T-cell therapy
-
Anti-CXCR5 CAR-T cells (green) attack lymphoma cells (magenta) within the stroma cell network of the B cell follicle (light blue).
© AG Höpken / Rehm, Max Delbrück Center