Vesicular transport
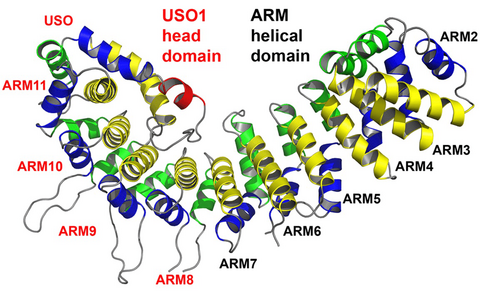
Vesicular transport in eukaryotic cells depends on conserved sets of proteins involved in the sequential steps of vesicle budding, uncoating, and tethering to the target membrane, as well as in membrane fusion and cargo release. Vesicle tethering at the target membrane is regulated by Rab/Ypt GTPases and involves both heteromultimeric tethering complexes and factors characterized by extended coiled-coil regions. We have extended earlier studies of the transport protein particle (TRAPP), a factor required for ER-to-Golgi transport by elucidating the crystal structure of the TRAPP-associated protein Tca17. For the golgin p115, whose crystal structure was determined earlier, we could eliminate a confusion as to the nature of the repeats forming the protein structure by proving unequivocally that p115 is made up of armadillo repeats and not of a new kind of repeats typical for tethering protein (Figure).
Armadillo fold of the human golgin p115. (A) The globular head region of p115, p115GHR, is composed of canonical armadillo repeats (ARM2 to ARM10). An unsual repeat, ARM11, contains an additional α-helix (USO) that folds into the concave surface of p115. (B) Superposition of the canonical armadillo repeats of p115 (left) and of β-catenin (right). The structure analysis proves that p115 is composed of regular armadillo repeats and not of a new type of repeats typical for vesicle tethering factors.
Figure adapted from
Striegl, H., Andrade-Navarro, M.A., Heinemann, U.
Armadillo motifs involved in vesicular transport
PLoS ONE 5 (2): e8991 (2010-02)