An animal that runs on hybrid fuel
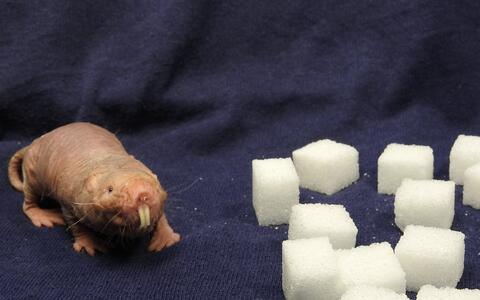
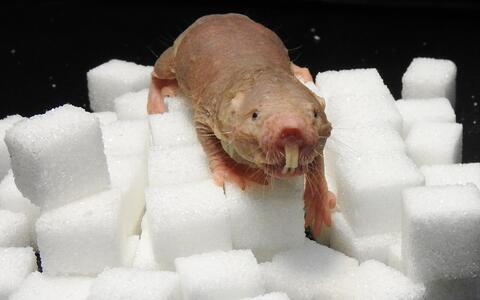
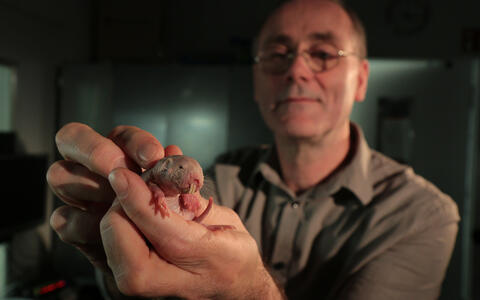
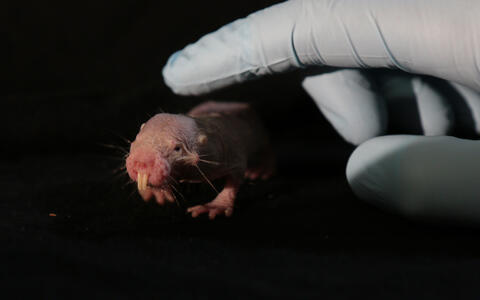
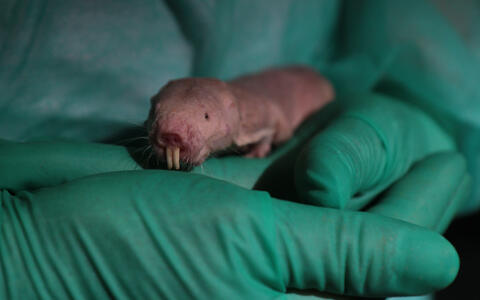
The naked mole-rat, a rodent native to Africa, can survive with little or no oxygen far longer than other mammals. The secret lies in its metabolism: in addition to the basic system by which animals generate energy from glucose, naked mole-rats have a backup system based on fructose. This discovery comes from Gary Lewin's lab at the Max Delbrück Center (MDC), in collaboration with scientists from around the globe. The work is published in the latest edition of the journal Science.
Oxygen is so essential to life that a very short deprivation is fatal to animals. Their cells need a constant supply to drive the chemical reactions that produce energy from food. In ancient times, cells evolved a form of metabolism that used the sugar glucose as a source of fuel and the high reactivity of oxygen atoms to extract its energy. This process was so efficient that glucose-based metabolism could fuel the bodies of humans and even larger animals, and it has been maintained over the course of evolution.
But life in a harsh environment can alter even very basic aspects of an animal's biology. Long ago, something drove the ancestors of the naked mole-rat underground. There the rodent's biology and behavior began an evolutionary dialogue with the extreme conditions it encountered. This led to some highly unusual adaptations. Naked mole-rats are insensitive to some forms of pain, and have lifespans that exceed 32 years – ten times the norm for most other rodents. Only one or two cases of cancer have ever been detected in the species. And now MDC scientists have discovered that the animal can go with little or no oxygen for extraordinary lengths of time.
Such characteristics have attracted the interest of scientists around the globe – including neurobiologist Gary Lewin. Over several years, his laboratory has gained deep insights into the biology of pain by comparing the nervous system of the naked mole-rat to that of mice and humans. Upon learning that the naked mole-rat could cope with little or no oxygen, he was immediately intrigued – and his lab was well prepared to pursue the biology behind this unique attribute.
Linking oxygen deprivation to a unique metabolic system
Oxygen deprivation was clearly connected to the animal's biology, lifestyle and environment. "Naked mole-rats huddle in huge, underground colonies of up to 280 individuals," Lewin says. "This means that they continually experience sharp declines in levels of oxygen and dramatic increases in carbon dioxide. Without adaptations, this would be just as deadly to the naked mole-rat as it is to other animals."
Most organisms on Earth are suited to the surface atmosphere, composed of about 21% oxygen and only tiny amounts of carbon dioxide (about 0.04%). Reducing oxygen to about 5% is fatal for a mouse within about 15 minutes; total deprivation causes fatal damage within about a minute. The naked mole-rat, however, can cope with as little as 5% oxygen and high levels of carbon dioxide for hours on end with no apparent distress or ill effects. And amazingly, it can survive at least 18 minutes without any oxygen at all.
"Under these conditions the animal enters a sort of suspended animation," says Jane Reznick, a postdoc in Lewin's group and a lead author on the current paper. "It falls asleep and its heartbeat slows to about a quarter of the normal rate. When oxygen is restored the heart rate rises, and the animal quickly wakes up and goes about its normal behavior."
This hinted that some backup system was protecting its heart and brain – two organs that are highly sensitive to oxygen in other species. Without it, their cells cannot produce energy and rapidly suffer fatal damage.
Hitting the stop button on an assembly line
There had to be some fundamental difference in the naked mole-rat's metabolism. To find it, the scientists enlisted help from the MDC's Metabolomics Unit, headed by Stefan Kempa. His team uses advanced technology to capture global and quantitative snapshots of cellular metabolism. Their methods reveal the presence of tiny metabolites: molecules that are created through the processing of fuels like glucose. Networks of enzymes break glucose down into small products that move through the metabolic pipeline, generating energy along the way.
"These experiments are a bit like hitting the 'stop' button on an assembly line," Kempa says. "If you were to do that in a factory, then look at partially assembled pieces and the bits that were tossed out, you'd get an idea of what was being built, and how it was constructed." Further experiments traced the remnants of the sugars as they flowed through an alternative metabolic route that generated energy without consuming oxygen.
Comparing mouse and naked mole-rat tissues under conditions with and without oxygen revealed some curious differences. In naked mole-rats, oxygen deprivation triggered a shutdown of cellular energy factories called mitochondria. In the mouse they continued to operate but quickly malfunctioned – mitochondria need oxygen to run.
But the most startling finding had to do with the sugar molecules found in the animals' blood and tissues. Overall, naked mole-rats had a lot less glucose than mice, which hinted that other sugars might be providing an alternative source of energy. During oxygen deprivation, there was a significant rise in levels of other sugars. Naked mole-rats had more sucrose – and truly stunning was the amount of fructose, which had skyrocketed.
Can neurons function if they feed exclusively on fructose?
Could the naked mole-rat be using fructose rather than glucose as a source of energy? The two sugars weren't that different – even our own bodies make use of fructose-based metabolism, although this only happens in the kidney and liver. These organs have an enzyme called ketohexokinase, or KHK, which can trim fructose into a form that can be plugged into the energy production line. From that point on the modified fructose, called fructose-1-phosphate (F1P), is handled like glucose. Since the subsequent stages of processing don't require oxygen, it wouldn't be absolutely necessary in a metabolic system based on fructose.
"In humans, fructose metabolism occurs only in the kidney and liver because they're the only tissues that contain KHK," Lewin says. "We found that brain tissue from the naked mole-rat contained high levels of F1P – suggesting that KHK was at work – but only under oxygen deprivation. This told us two things: that their brains might really be using fructose as a source of energy, and that the switch only happened when oxygen grew scarce."
The evidence for fructose metabolism was accumulating, but so far it was all indirect; the next step would be to determine whether the animals were actually using the alternative source of fuel. First the scientists performed experiments using brain tissue to test whether neurons could function if they were deprived of glucose and fed exclusively on fructose. While an hour of this treatment severely damaged the cells of mice, naked mole-rat neurons continued to show activity. Experiments carried out in Michael Gotthardt’s group showed even more dramatic results for the naked mole-rat heart, which could beat just as well when supplied with fructose as it could using glucose.
"This was proof that fructose can replace glucose as an energy source in the naked mole-rat brain and heart," Reznick says. "It helps explain how these organs – and the animal as a whole – can recover from long periods of oxygen deprivation."
A two-part system for switching to alternative fuel
Cells can only use fructose as an energy source if they can absorb it from their surroundings. This requires a protein called GLUT5, which snatches fructose and draws it into the cell. In mice and humans, GLUT5 appears in kidney and liver cells, but other tissues have almost none. It's another factor that restricts fructose metabolism to the kidney and liver in humans and prevents it from serving vital organs such as the brain and heart. In the naked mole-rat those tissues – and most other cells – have at least ten times as much GLUT5.
"This gives the naked mole-rat a two-part system that allows it to survive long periods of oxygen deprivation," Lewin says. "Throughout its body you find both the GLUT5 transporter and the KHK enzyme that converts fructose into a usable energy source."
Fructose metabolism has been encountered in human diseases including malignant cancer, metabolic syndrome, and heart failure. This hints that there might be some link between the naked mole-rat's metabolism, its resistance to cancer, and possibly even its extraordinary lifespan. Only further research will tell – but the current study provides an interesting new handle on such questions.
"It's important to understand how these unusual animals make the metabolic switch without any obvious long-term damage to their tissues," Lewin says. "We might learn something about how our own cells attempt to cope with situations in which they are deprived of oxygen, such as strokes or heart attacks. Our work raises questions about the biology of fructose metabolism that will 'fuel' research for years to come."
Images: Roland Gockel / MDC
Thomas J. Park1, Jane Reznick2, Bethany L. Peterson1 , Gregory Blass1 , Damir Omerbašić2, Nigel C. Bennett3, P. Henning J.L. Kuich4, Christin Zasada4, Brigitte M. Browe1, Wiebke Hamann5, Daniel T. Applegate1, Michael H Radke5,10, Tetiana Kosten2, Heike Lutermann3, Victoria Gavaghan1, Ole Eigenbrod2, Valérie Bégay2, Vince G. Amoroso1, Vidya Govind1, Richard D. Minshall7, Ewan St. J. Smith8, John Larson9, Michael Gotthardt5,10, Stefan Kempa4, Gary R. Lewin2,11 (2017): „Fructose driven glycolysis supports anoxia resistance in the naked mole-rat.“ Science. doi:10.1126/science.aab3896
1Laboratory of Integrative Neuroscience, Department of Biological Sciences, University of Illinois at Chicago, Chicago, Illinois, United States of America; 2 Molecular Physiology of Somatic Sensation, Max Delbrück Center for Molecular Medicine, Berlin, Germany; 3Department of Zoology and Entomology, University of Pretoria, Pretoria, Republic of South Africa; 4Integrative Proteomics and Metabolomics, Berlin Institute for Medical Systems Biology, Max Delbrück Center for Molecular Medicine, Berlin, Germany; 5Neuromuscular and Cardiovascular Cell Biology, Max Delbrück Center for Molecular Medicine, Berlin, Germany; 7Departments of Anesthesiology and Pharmacology, University of Illinois at Chicago, Chicago, Illinois, United States of America; 8Department of Pharmacology, University of Cambridge, Cambridge, United Kingdom; 9Department of Psychiatry, University of Illinois at Chicago, Chicago, Illinois, United States of America; 10DZHK partner site Berlin, Germany; 11Excellence cluster Neurocure, Charité Universitätsmedizin Berlin, Germany