New differentiation switch identified in intestine
“The intestine is a fantastic organ for researching the interplay among different signaling pathways”, says Dr. Julian Heuberger of the largest human organ. What’s special about our gut, which can be up to eight meters long, is its surface area of 400 to 500 m2. This large surface area is achieved because of the microscopically small finger-like extensions of the mucosal cells (the “brush border” or microvilli), invaginations of the mucosa into the underlying connective tissue (the “crypts of Lieberkuhn”) as well as, in the small intestine, by projections of the mucosa into the lumen (the intestinal villi) and the formation of folds (valves of Kerckring). The extremely high regeneration rate of the intestinal epithelium is also noteworthy. It renews itself completely every four to five days. During this process, all the epithelial cell types are formed from the stem cells in the crypts by means of differentiation processes. In addition to the cells that take up nutrients (absorptive enterocytes), these differentiated epithelial cells also contain cells that release substances (secretory cells) like the goblet, tuft, paneth and enteroendocrine cells.
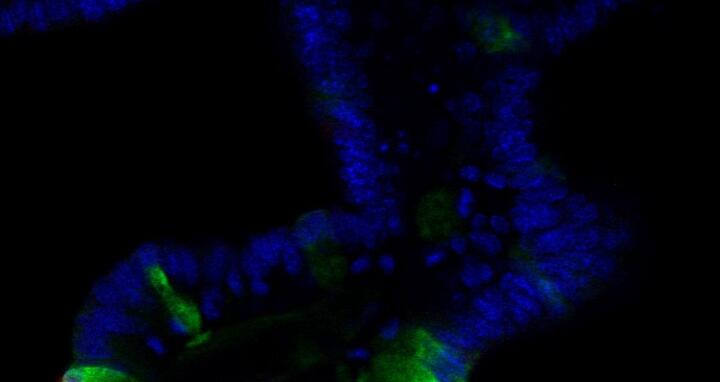
But how do the different epithelial cell types form from the stem cell population in a fast, organized and controlled fashion? “In the intestinal crypts, the activation of different signaling pathways leads to different forms of cell differentiation or to preservation of the stem cells. The individual signaling pathways have been relatively well researched, but certain pieces of the puzzle are still missing”, explains Heuberger. Working with Prof. Walter Birchmeier, Heuberger found one of these pieces in early 2014. These two scientists and their team showed for the first time that a signaling pathway, via activation of tyrosine phosphatase Shp2 (src homology 2 domain-containing protein tyrosine phosphatase 2) and the subsequently mediated MAPK (mitogen-activated protein kinase) signal pathway, helps decide whether a precursor cell becomes a goblet cell or a paneth cell. For the study published in PNAS, they used both mouse models and in vitro organoid systems that “constitute an excellent supplement to traditional animal models for certain questions”, says Heuberger.
The Shp2 mouse model and its phenotype
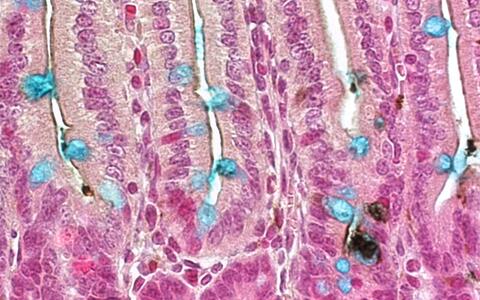
Previous studies by other research groups indicated to the MDC scientists that the tyrosine phosphatase Shp2 could be a switch for intestinal cell differentiation. In order to investigate Shp2’s functions in the intestine, the scientists used conditional mutagenesis to create a special strain of mouse that cannot form Shp2 in its intestinal epithelium and is thus Shp2-deficient. “The phenotype of these mice was clear”, explains Heuberger. “The Shp2 mutants showed drastically reduced numbers of goblet cells in their small and large intestines and a dramatic increase in the number of paneth cells.” This led, as the animals aged, to colitis and retarded growth.
The “mini-intestines” and their contribution to the project
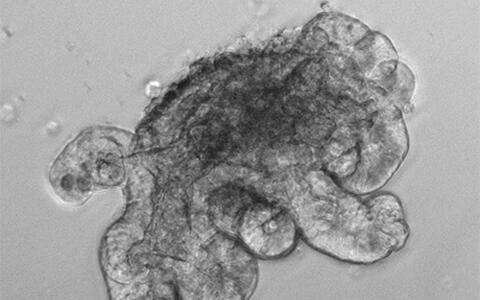
“Using our in vitro organoid system, we were able to test very well for a potential direct relationship between Shp2 signaling and paneth cell differentiation”, reports Heuberger. “Our intestinal organoids are, to put it simply, “mini-intestines”, but without connective tissue and immune cells. These are purely epithelial systems that we grow from isolated intestinal crypts in a cell culture.” Because of this purely epithelial nature, the organoids simplify investigation of intrinsic biochemical or differentiation processes of the epithelium even though they are quite an artificial system. Removal of Shp2 from the organoid cultures led to an increased number of paneth cells. “This was the direct evidence that the greater number of paneth cells that we had previously identified in the mice was a primary and not a secondary effect resulting, for example, from the colitis.”
Earlier studies had shown the importance of the canonical Wnt signal transduction pathway, which is mediated by the β-catenin molecule interacting with the transcription factor TCF4, for paneth cell differentiation. A genome-wide expression analysis of the Shp2-deficient mouse intestines conducted by Heuberger and his team showed that the Wnt/β-catenin signaling pathway is indeed activated to a greater extent in these animals. In addition to various Wnt ligands and target genes, this analysis also showed that the stem-cell-typical Lgr5 gene (leucine-rich repeat-containing G-protein-coupled receptor 5) is formed to a greater extent in the Shp2-deficient mice. “Further analyses confirmed this and show that other stem cell genes are also up-regulated in the Shp2 mutants. It became clear in the end that these mice display not only increased paneth cell differentiation but also an expansion of the stem-cell niche.”
Explanation of the signaling mechanism
What signaling pathway does Shp2 regulate in the processes observed, and how is Wnt signaling influenced? These were the researchers’ main questions at the time. “A hot candidate that is regulated by Shp2 in many cell types is the MAPK signaling pathway via the MEK1 kinase (mitogen-activated protein kinase, MAP2K1), which in turn activates two additional kinases, namely ERK1 and 2 (mitogen-activated protein kinases 1 & 3, MAPK3 & MAPK1). We have now been able to show this for the intestine as well.”
The Shp2-deficient mice display a drastically reduced level of activated ERK1 & 2”, Heuberger explains. Additional experiments, including preparation of a Shp2-deficient and MEK1-activated double-mutated mouse strain, helped reinforce this theory. These double mutants, generated in collaboration with Klaus Rajewsky, displayed goblet cell numbers similar to those of the control animals plus a drastically reduced number of paneth cells. This means that the additionally introduced activated MEK1 kinase had reversed the Shp2 goblet cell phenotype and had completely prevented paneth cell differentiation. In addition, these animals showed a reduction in the size of the stem-cell niche.
As the last portion of their analyses, the scientists demonstrated that MEK1-based regulation of Wnt activity in the differentiation of paneth and goblet cells occurs post-translationally via an alteration in the stability of a particular form of TCF4. The focus was on two subtypes differing in gene activation potentials, with Shp2/MEK1 activity altering the ratio between the two. The scientists demonstrated that Shp2/MEK1 activity stabilizes the less gene-activating form of TCF4 by influencing protein degradation in the proteasome. In summary, Heuberger explains: “In this study, we showed that the interplay between Wnt and Shp2/MAPK signaling pathways in a precursor cell population in the intestinal crypts regulates specialization or differentiation into goblet or paneth cells. If Wnt signaling is predominant in further differentiation, a switch is made toward paneth cells, while increased goblet cell differentiation occurs if Shp2-MAPK signaling predominates.
Ongoing utilization of the organoids
“In my current research, I use the organoid system to analyze effects of oncogenes and potential tumor-supporting genes. This gives me first indications as to whether a particular molecule plays a role in cell division and/or differentiation, as well as in the genesis of cancers. It is a great way to test the potential relevance of new intestinal animal models in advance”, reports Heuberger. These altered organoids provide him with the perfect tool for investigating how tumor cells activate particular gene networks.
Featured Image: Julian Heuberger in his lab. Photo: Susann Förster/MDC