The harmful effects of immune cells in hypertension
Hypertension, or high blood pressure, tops the list of chronic health conditions. It affects about one-third of the world’s population, including nearly 44 percent of German citizens. If the pressure in the blood vessels is too high, the body’s organs – mainly the brain, the heart, and the blood vessels – suffer as a result. The consequences go beyond an increased risk of developing serious cardiovascular diseases like strokes or heart attacks. In a healthy body, the heart, brain, and blood vessels also play a key role in regulating blood pressure. If they are damaged by persistently high blood pressure, this regulatory ability is lost – creating a vicious circle.
Conventional medications can lower blood pressure, but they fail to achieve the desired protective effect on the organs in a large portion of patients.
To lower blood pressure, patients should make changes to their lifestyle, such as eating a well-balanced, low-salt diet, exercising regularly, and stopping smoking. Some drugs, like beta blockers and ACE inhibitors, can also help: “Conventional medications can lower blood pressure, but they fail to achieve the desired protective effect on the organs in a large portion of patients,” says Dr. Suphansa Sawamiphak, who heads the Cardiovascular-Hematopoietic Interaction Lab at the Max Delbrück Center. This is particularly evident, she says, in the brain, where hypertension causes tiny blood vessels to become permeable, or eventually die off, adding: “This means there must be other control centers in the overall process that we can’t target with conventional therapeutic agents.”
Researchers have known for some time that components of the immune system may play a role here. Inflammatory responses in the body contribute to high blood pressure and have harmful effects on organs, but it is not yet known exactly how this occurs.
Immune cells damage blood vessels in the zebrafish brain
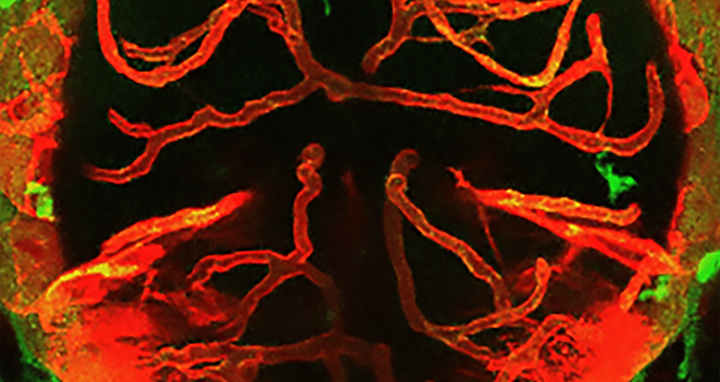
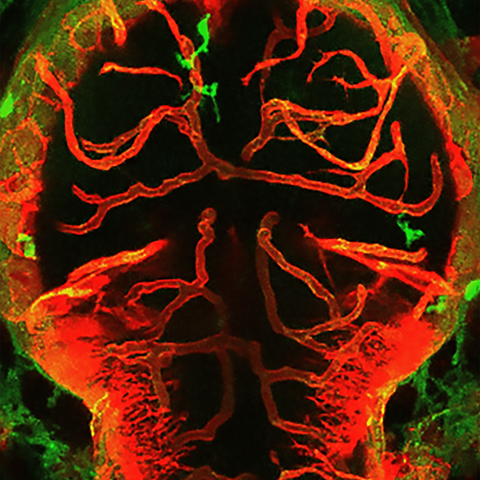
Interactions between macrophages/microglia and cerebral vessels lead to vascular regression and cell death in the brain. Image depicting the brain of a zebrafish with macrophages/microglia labeled in green and blood vessels in red.
So Sawamiphak and her team at the Max Delbrück Center and collaborators working in Italy and Switzerland, studied larval zebrafish to shed more light on the underlying biological mechanisms. “This is an excellent model system for investigating many questions, since it is easy to manipulate the organisms by changing the environment,” explains the biologist, adding: “Because young zebrafish are transparent, we can literally see how this affects the living fish.”
To analyze the role of the immune system in hypertension, the research team raised zebrafish larvae in water with low ion concentration. This creates an ion imbalance in their bodies that is comparable to excessive salt consumption in humans, thus leading to high blood pressure. The team then examined how this affects the blood vessels in the brain.
Our findings suggest that macrophages and microglia undergo extensive reprogramming during hypertension.
According to the researchers’ observations, hypertension causes an increase in both the number of macrophages and microglia – special immune cells of the brain – that can get in touch with the vascular surface. There, they come into contact with the endothelium, the innermost cellular layer of blood vessels, and progressively weaken the vessel walls. Damage is also done to the blood-brain barrier, which prevents harmful substances and pathogens in the blood from reaching the brain. “The interesting thing is that when blood pressure levels are healthy, macrophages and microglia normally help protect the vessels,” says Sawamiphak. “Our findings suggest that macrophages and microglia undergo extensive reprogramming during hypertension.”
Blocking signaling molecules prevents organ damage
An important role is played by inflammatory messengers like interferon gamma, which are released at a higher rate under hypertensive conditions. To experimentally substantiate this connection, they switched off the gene for a receptor to which interferon gamma normally binds. In these fish, hypertension did not cause any damage to blood vessels or to the blood-brain barrier. The team also succeeded in demonstrating in mice that therapeutic agents that inhibit interferon gamma can prevent common side effects of hypertension – including damage to the blood-brain barrier, degradation of blood vessels in the brain, and cognitive deficits.
“Our findings provide a completely new perspective on the role of inflammatory processes in the progression of hypertension,” says Sawamiphak, explaining the significance of her work. Now, she says, it is necessary to more precisely characterize the immune cells and immunomodulators involved in such processes and to verify their role in higher animals including humans. If this can be confirmed, it would mean that the team had uncovered new therapeutic targets for hypertension through this study. This would particularly benefit patients for whom conventional drugs have failed to protect against progressive organ damage.
Text: Stefanie Reinberger
Further information
Literature
Dilem C Apaydin, Bhakti I Zakarauskas-Seth et al. (2023): “Interferon-γ drives macrophage reprogramming, cerebrovascular remodeling, and cognitive dysfunction in a zebrafish and a mouse model of ion imbalance and pressure overload.”; Cardiovascular Research; doi: 10.1093/cvr/cvac188
Downloads
Interactions between macrophages/microglia and cerebral vessels lead to vascular regression and cell death in the brain. Image depicting the brain of a zebrafish with macrophages/microglia labeled in green and blood vessels in red. Picture: Maria P. Kotini, University of Basel
Portrait of Dr. Suphansa Sawamiphak. Picture: Felix Petermann, Max Delbrück Center
Contacts
Dr. Suphansa Sawamiphak
Head of the Cardiovascular-Hematopoietic Interaction Lab
Max Delbrück Center
+49-(0)30-94060-3680
Suphansa.Sawamiphak@mdc-berlin.de
Christina Anders
Editor, Communications Department
Max Delbrück Center
+49-(0)30-9406-2118
christina.anders@mdc-berlin.de or presse@mdc-berlin.de
- Max Delbrück Center
-
The Max Delbrück Center for Molecular Medicine in the Helmholtz Association (Max Delbrück Center) is one of the world’s leading biomedical research institutions. Max Delbrück, a Berlin native, was a Nobel laureate and one of the founders of molecular biology. At the locations in Berlin-Buch and Mitte, researchers from some 70 countries study human biology – investigating the foundations of life from its most elementary building blocks to systems-wide mechanisms. By understanding what regulates or disrupts the dynamic equilibrium of a cell, an organ, or the entire body, we can prevent diseases, diagnose them earlier, and stop their progression with tailored therapies. Patients should be able to benefit as soon as possible from basic research discoveries. This is why the Max Delbrück Center supports spin-off creation and participates in collaborative networks. It works in close partnership with Charité – Universitätsmedizin Berlin in the jointly-run Experimental and Clinical Research Center (ECRC), the Berlin Institute of Health (BIH) at Charité, and the German Center for Cardiovascular Research (DZHK). Founded in 1992, the Max Delbrück Center today employs 1,800 people and is 90 percent funded by the German federal government and 10 percent by the State of Berlin.