How Cells Tolerate DNA Damage – MDC Researchers Identify Start Signal for Cell Survival Program
The DNA of
each human cell is damaged many times every day. DNA lesions can be caused by
ultraviolet radiation, errors in cell division, DNA-damaging chemicals or intracellular
metabolic products. Damaged chromosomal DNA can ultimately be the cause for
serious diseases, such as cancer.
However,
cells have developed complex systems that recognize DNA lesions within seconds
and ensure that the damage will be repaired. In case of massive DNA damage, the
affected cell can be destroyed by initiating apoptosis (programmed cell death).
Apoptosis
is a cellular program which drives defective cells to commit suicide, thus
protecting the organism as a whole. Another regulator of gene expression, the
transcription factor p53 – also known as the “guardian of the genome” – has a
key function in the activation of programmed cell death. But p53 is not always
successful in switching on the protective program.
NF-kappaB
opposes the function of p53, in turn activating a survival program which
protects the damaged cells from destruction. The activation of this program by
NF-kappaB is considered to be one of the possible causes for resistance of
tumor cells to chemo or radiation therapy.
The
transcription factor NF-kappaB not only regulates cell survival programs, it
also plays an important role in the immune system and in inflammatory
processes. NF-kappaB can be switched on by a number of extracellular and
intracellular stimuli.
Such
stimulation alters the activity of protein-regulated signaling pathways, which
ultimately activate NF-kappaB. The process of signal transduction initiated by
external physiological stimuli has been well characterized in recent years.
However,
it was not understood how NF-kappaB is switched on by DNA damage. MDC
researchers have now succeeded in illuminating this particular signaling
pathway.
Professor
Scheidereit and his colleagues Dr. Stilmann and Dr. Hinz discovered that the
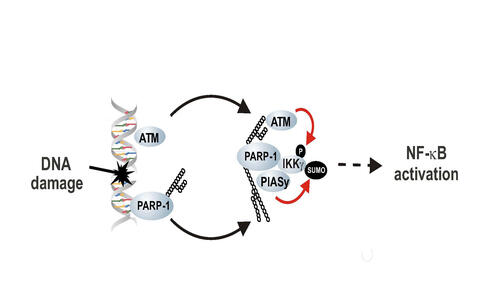
DNA damage detector PARP-1 plays a key role in the activation of NF-kappaB.
PARP-1 recognizes sites of DNA damage within seconds and then attracts several
proteins, which are important for this signaling pathway, to form a complex in
the cell nucleus.
Due to
subsequent chemical changes in the complexed proteins, signals are generated,
which then trigger NF-kappaB activation. “We have thus identified the start
signal for NF-kappaB activation”, Dr. Stilmann and Dr. Hinz explained.
Now the
researchers want to investigate further components of this signal transmission
cascade and their interaction. “For medical research it is of enormous
significance to understand these signaling pathways. This is based on the hope
to identify targets for the development of drugs, which allow to switch off the
survival factor NF-kappaB in cancer diseases in a context-specific manner.”
Already
now, clinical trials are in progress worldwide with various, not yet approved
substances, which target and inhibit PARP-1 and which have gained much attention
from the scientific community. In the context of these studies, experts
consider the work of the MDC researchers to be of special significance.
Professor
Scheidereit and his collaborators have been working for many years on
NF-kappaB. Some years ago they were able to show that NF-kappaB plays a key
role in tumor cell survival in Hodgkin’s lymphoma, a common lymph gland cancer.
*A nuclear Poly(ADP-ribose)-dependent signalosome confers DNA damage
induced IκB kinase activation
Michael
Stilmann1,2*, Michael Hinz1*,
Seda Çöl Arslan1,3, Anja Zimmer1,
Valérie Schreiber4 and
Claus Scheidereit1
1Max DelbrückCenter for Molecular Medicine, Robert-Rössle-Strasse
10, 13125 Berlin, Germany;
2FreeUniversity, Faculty of Biology, Chemistry and
Pharmacy, Takustr. 3, 14195 Berlin; 3Humboldt University, Institute of Biology,
Chausseestrasse 117, 10115 Berlin; 4Université Strasbourg 1, Institut Gilbert Laustriat,
CNRS - UMR 7175, Département Intégrité du Génome, ESBS, Bld Sébastien Brant, BP
10413, Illkirch Cedex, France
* These authors contributed equally to this work
MDC researchers have shown that the sensor protein PARP-1, activates the transcription factor NF-kappaB, a survival factor for cancer cells. (Graphic: Michael Hinz/Copyright: MDC)
Barbara Bachtler
Press and Public Affairs
Max Delbrück Center for Molecular Medicine (MDC) Berlin-Buch
Robert-Rössle-Straße 10; 13125 Berlin; Germany
Phone: +49 (0) 30 94 06 - 38 96
Fax: +49 (0) 30 94 06 - 38 33
e-mail: presse@mdc-berlin.de
http://www.mdc-berlin.de/
Further information:
Stephen P. Jackson, Jiri Bartek, The DNA-damage response in human biology and disease, Nature Vol. 461, October 22, 2009
Peter C. Fong et al., Inhibition of Poly(ADP-Ribose) Polymerase in Tumors from BRCA Mutation Carriers ; New England Journal of Medicine, Vol. 361, Nr. 2, pp. 123-134, July 9, 2009