Infante Duarte Lab
Innate Immunity & Neuroinflammation
Profile
Chronic inflammation of the central nervous system (CNS) is caused by sustained activation of CNS cells such as microglial and astrocytes, and recruitment and activation of other peripheral and tissue-resident immune cells within the brain. This persistent inflammation can lead to progressive brain damage, impaired neuronal function and eventually to severe disability and cognitive weakening.
The Infante-Duarte lab focuses on the pathophysiology of chronic CNS damage, in particular in multiple sclerosis (MS) and neuromyelitis optica spectrum disorders (NMOSD). We want to understand how components of the immune system contribute to CNS inflammation and neurodegeneration. Moreover, we investigate how brain inflammation affects structures of the brain extracellular matrix and develop matrix-related non-invasive imaging tools (principally MR-based approaches) for monitoring progression and therapy response in neuroinflammation.
Team
Group Leader
Prof. Dr. Carmen Infante Duarte
carmen.infante@charite.de
PhD Students
Alba Del Rio Serrato
alba.del-rio-serrato@charite.de
Maria E. Schröder
maria-eugenia.schroeder@charite.de
Rafaela Vieira da Silva
rafaela.vieira-da-silva@charite.de
MD/ MD-PhD Students
Moataz Alabdullah
moataz.alabdullah@charite.de
Lina Anderhalten
lina-carlotta.anderhalten@charite.de
Clara Batzdorf
clara.batzdorf@charite.de
Julian Bossenmaier
julian.bossenmaier@charite.de
Technical Assistent
Natalie Asselborn
natalie.asselborn@charite.de
Bibiane Seeger
bibiane.seeger@charite.de
Research
- Innate lymphoid cells in the healthy and inflamed brain
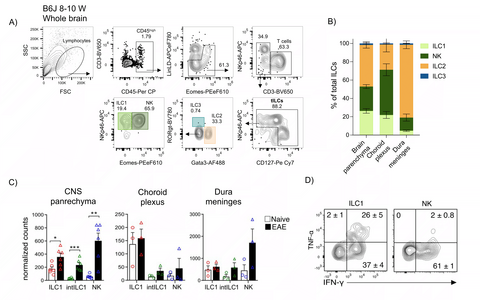
Innate lymphoid cells (LCs) constitute a new family of innate immune cells that act as important modulators of the immune response. The ILC compartment comprises a highly heterogeneous group of cells that have been divided into five subsets based on their developmental pathways and their phenotypical and functional profiles: cytotoxic NK cells, helper-like ILC1, ILC2 and ILC3s and LTi cells.
We have shown evidence for deficient NK cell activity in patients with MS, suggesting that NK cells may have a protective, disease-limiting role in neuroinflammation. However, both beneficial as well as detrimental roles for NK cells have been found in studies using EAE. Since former studies made no distinction between the heterogeneous group 1 ILC compartment that includes NK and also ILC1, we further hypothesized that the apparent conflicting results may also originate from the lack of proper discrimination between the different ILC subtypes. Although ILCs have been reported to be present predominantly in barrier and mucosal tissues, we and others have reported that in steady-state conditions, the adult CNS also contains infiltrating and resident ILCs (predominantly ILC2s and group 1 ILCs). These ILCs are located in both, parenchymal and non-parenchymal structures, and display a strict tissue compartmentalization (parenchyma, perivascular spaces, subdural meninges and choroid plexus). Furthermore, these ILCs seem to start infiltrating the brain during early stages of development and do not re-circulate.
Thus, our line of research focuses on the study of the formation and heterogeneity of the CNS-ILC compartment, and its role in the development and maintenance of tissue homeostasis of the CNS. Furthermore, we study the immunomodulatory roles of ILCs in the context of neuroinflammation as the one observed during MS and its experimental autoimmune mouse model, the EAE.
Figure 1: A, B) Innate lymphoid cells populate the healthy mouse brain, differentially distributed along distinct CNS compartments (CNS parenchyma, choroid plexus and dura meninges), with group 1 ILCs and ILC2s as the main subsets. C) Group 1 ILCs increased within the brain parenchyma during neuroinflammation and D) ILC1 differ from NK cells in the production of immunomodulatory cytokines such as TNFα and IFNγ.
Selected Publications
- Hamann, I., et al., Characterization of natural killer cells in paired CSF and blood samples during neuroinflammation. J Neuroimmunol, 2013. 254(1-2): p. 165-9.
- Hertwig, L., et al., CX3CR1-dependent recruitment of mature NK cells into the central nervous system contributes to control autoimmune neuroinflammation. Eur J Immunol, 2016. 46(8): p. 1984-96.
- Romero-Suarez, S., et al., The Central Nervous System Contains ILC1s That Differ From NK Cells in the Response to Inflammation. Front Immunol, 2019. 10: p. 2337.
- Schwichtenberg, S.C., et al., Fingolimod Therapy in Multiple Sclerosis Leads to the Enrichment of a Subpopulation of Aged NK Cells. Neurotherapeutics, 2021. 18(3): p. 1783-1797.
- Immune alterations in NMOSD and MOGAD
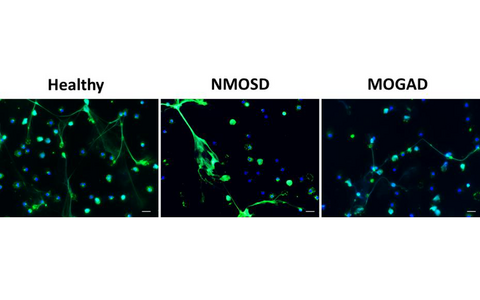
Neuromyelitis optica spectrum disorders (NMOSD) are a group of severe antibody-mediated autoimmune diseases of the CNS, long believed to be rare variants of MS. Serum autoantibodies against aquaporin-4 (AQP4-IgG) are found in ≥ 80 % of NMOSD patients. Around 10-40% of AQP4-IgG seronegative NMOSD patients are seropositive for autoantibodies against myelin oligodendrocyte glycoprotein (MOG-IgG) and suffer from the newly described (MOG)-associated disease (MOGAD). Increasing evidence suggests a contribution of both innate and adaptive immune components to the inflammatory cascade inside the CNS. In this line, we previously showed that NMOSD neutrophils display deficient functionalities such as reduced fMLP-induced neutrophil migration and oxidative burst.
Currently, we are investigating immune alterations, in particular affecting B cell and neutrophil functionality, that may contribute to the pathophysiology of NMOSD and MOGAD. We hypothesized that neutrophils from patients may have a deficient response to death stimuli and, therefore, accumulate within the brain tissue and contribute to the inflammation. In our more recent project, cell death of neutrophils from patients and healthy controls are being investigated in an in-vitro set-up.
Figure 1: Images of neutrophils from healthy people as well as from AQP4-IgG seropositive NMOSD and MOGAD patients that were stimulated in vitro with PMA to induce cell death. Living as well as apoptotic and NETotic neutrophils are shown. Immunofluorescence staining of DNA in blue and neutrophil elastase (NE) in green represent cell nuclei and NETotic neutrophils, respectively. Images were acquired using an inverted fluorescent microscope at 40× magnification; scale bar 20µm.
Selected Publication
Hertwig, L., et al., Distinct functionality of neutrophils in multiple sclerosis and neuromyelitis optica. Mult Scler, 2016. 22(2): p. 160-73.
- Investigation of the disease-related alteration of the extracellular matrix in a mouse model of multiple sclerosis using molecular and biophysical MRI
The extracellular matrix (ECM) is a complex and specialized compartment composed of a network of glycosaminoglycans (GAGs), proteoglycans and glycoproteins that mediates diverse cellular and molecular processes. In multiple sclerosis, the brain matrix has emerged as an important player in disease pathogenesis. Thus, we hypothesize that inflammation-related ECM alterations are excellent targets to in-vivo image tissue pathology in the course of autoimmune neuroinflammatory disorders, such as MS and its animal model of experimental autoimmune encephalomyelitis (EAE).
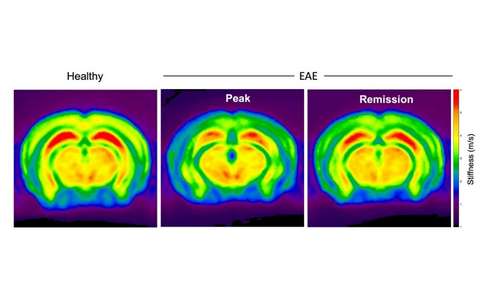
In our previous work, we showed that, during brain inflammation, very small superparamagnetic iron oxide nanoparticles (VSOP) bind to modified GAGs on activated brain endothelial cells and ECM. Using the VSOP we also demonstrated that VSOP reveals pathological alterations in the choroid plexus. Furthermore, we showed that non-invasive multifrequency magnetic resonance elastography (MRE) detect and quantify in vivo neuropathological changes based on the biomechanical properties of brain tissue. Moreover, our more recent data indicated that MRE senses sex-specific features of the brain tissue as well as specific alterations of the CNS matrix during brain inflammation, which are also visualized by VSOP but not by gadolinium-based contrast agents.
Currently, we are investigating the nature and reversibility of ECM modifications occurring during neuroinflamation and the possibility to image those change in vivo by MRE and/or nanoparticle- or contrast agent-based MRI.
Figure 1: MR-Elasticity maps of healthy and EAE brains. Reduced brain stiffness (c in m/s) at peak phase of EAE, a multiple sclerosis model, is partially recovered during the remission phase.
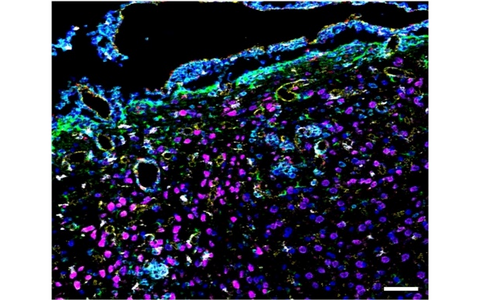
Figure 2: Histological detection of magnetic nanoparticles as well as immune and glia cells in the mouse brain using Imaging Mass Cytometry (IMC). Periventricular area with intense inflammatory infiltrate (CD45+ cells, cyan) and gliosis (GFAP+ cells, green; Iba1+ cells, white) promoting inflammation-mediated binding of magnetic particles (Eu-VSOP, red), particularly associated with endothelium (CD31+ cells, yellow).
Selected Publications
- Riek, K., et al., Magnetic resonance elastography reveals altered brain viscoelasticity in experimental autoimmune encephalomyelitis. Neuroimage Clin, 2012. 1(1): p. 81-90.
- Millward, J.M., et al., Iron oxide magnetic nanoparticles highlight early involvement of the choroid plexus in central nervous system inflammation. ASN Neuro, 2013. 5(1): p. e00110.
- Millward, J.M., et al., Tissue structure and inflammatory processes shape viscoelastic properties of the mouse brain. NMR Biomed, 2015. 28(7): p. 831-9.
- Berndt, D., et al., Inflammation-induced brain endothelial activation leads to uptake of electrostatically stabilized iron oxide nanoparticles via sulfated glycosaminoglycans. Nanomedicine, 2017. 13(4): p. 1411-1421.
- Wang, S., et al., Increased Retention of Gadolinium in the Inflamed Brain After Repeated Administration of Gadopentetate Dimeglumine: A Proof-of-Concept Study in Mice Combining ICP-MS and Micro- and Nano-SR-XRF. Invest Radiol, 2019. 54(10): p. 617-626.
- Wang, S., et al., MR Elastography-Based Assessment of Matrix Remodeling at Lesion Sites Associated With Clinical Severity in a Model of Multiple Sclerosis. Front Neurol, 2019. 10: p. 1382.
- Silva, R.V., et al., Contribution of Tissue Inflammation and Blood-Brain Barrier Disruption to Brain Softening in a Mouse Model of Multiple Sclerosis. Front Neurosci, 2021. 15: p. 701308.
- Batzdorf, C.S., et al., Sexual Dimorphism in Extracellular Matrix Composition and Viscoelasticity of the Healthy and Inflamed Mouse Brain. Biology (Basel), 2022. 11(2).
- Mitochondrial alterations in the inflamed nervous system
Free radicals generated in the context of neuroinflammatory processes are known to cause mitochondrial damage and impair mitochondrial transport in axons. We therefore hypothesize that under inflammatory conditions and subsequent oxidative stress, defective transport and function of mitochondria may contribute to neuronal damage.
As part of this project, we investigate the influence of oxidative stress on the motility, morphology and functionality of axonal mitochondria in a model of ex vivo explanted spinal nerve roots and in acute brain sections.
We were able to show that oxidative stress leads to reduced axonal transport as well as to changes in mitochondrial morphology and functionality, both in the spinal root and the brain section models. Our investigations are now aimed at elucidating the mechanism of mitochondrial impairments as well as evaluating new treatment strategies for neuroprotection in our ex vivo models.
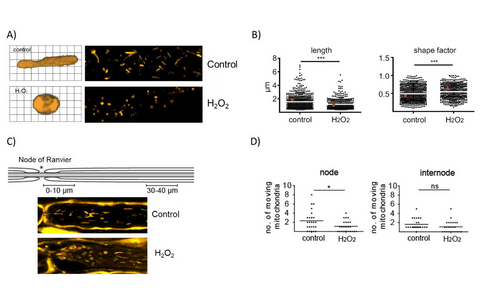
Figure 1: Effects of oxidative stress on morphology and motility of axonal mitochondria in a model of explanted peripheral nerves. A) and B) Oxidative stress induced by H2O2 exposure of explanted spinal nerve roots leads to changes in length and circularity (shape factor) of axonal mitochondria. C) H2O2-induced mitochondria alterations within the axons initiate at the nodes of Ranvier. D) Oxidative stress reduces mitochondria motility in the affected areas close to the nodes.
Selected publications
- Bros, H., et al., Oxidative damage to mitochondria at the nodes of Ranvier precedes axon degeneration in ex vivo transected axons. Exp Neurol, 2014. 261: p. 127-35.
- Bros, H., et al., Assessing Mitochondrial Movement Within Neurons: Manual Versus Automated Tracking Methods. Traffic, 2015. 16(8): p. 906-17.
- Bros, H., R. Niesner, and C. Infante-Duarte, An ex vivo model for studying mitochondrial trafficking in neurons. Methods Mol Biol, 2015. 1264: p. 465-72.
- Malla, B., et al., Teriflunomide preserves peripheral nerve mitochondria from oxidative stress-mediated alterations. Ther Adv Chronic Dis, 2020. 11: p. 2040622320944773.
- Malla, B., et al., Imaging and analysis of neuronal mitochondria in murine acute brain slices. J Neurosci Methods, 2022. 372: p. 109558.
- Malla, B., et al., Teriflunomide Preserves Neuronal Activity and Protects Mitochondria in Brain Slices Exposed to Oxidative Stress. Int J Mol Sci, 2022. 23(3).