How cells rewrite their fate
Joint press release by the Centre for Genomic Regulation (CRG) and the Max Delbrück Center for Molecular Medicine
Cell fate determines which cell type a cell differentiates into and which function it assumes in the organism. If cells make the wrong decision in this process, cancer can develop.
Researchers at the Centre for Genomic Regulation (CRG) in Barcelona and the Max Delbrück Center in Berlin have investigated how how cells can accelerate their fate. They present their results in the scientific journal “eLife”. The study could be the starting point for new methods to alter the molecular mechanisms of cancer development.
Let phagocytes off the leash
Central to the study is C/EBPα (CCAAT/enhancer-binding protein alpha), a protein that orchestrates the conversion of B lymphocytes to macrophages. B lymphocytes are white blood cells that form antibodies against pathogens. Macrophages also belong to the white blood cells. Called “scavenger cells”, they find and eliminate pathogens and pathologically altered cells. C/EBPα is a transcription factor, a type of protein which binds to specific DNA sequences in the regulatory regions of genes where it activates or represses protein expression.
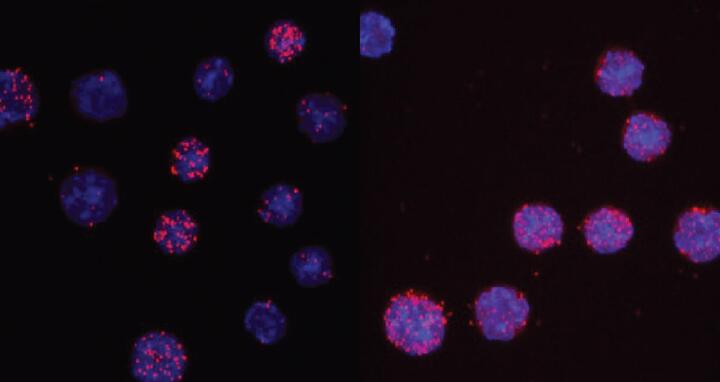
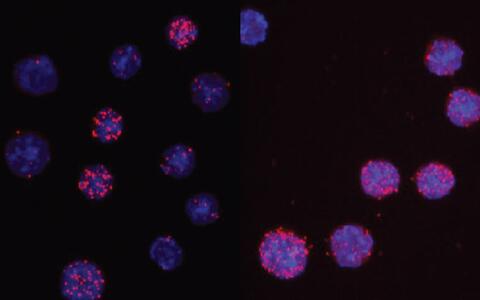
C/EBPαR35A interacts more strongly with PU.1 than unmated C/EBPα. The images show the nuclei of cells in light blue and the interaction readout as red dots. For the wild type only 3 of 13 cells show signals, while for the mutant 7 of 8 cells are positive.
Like all proteins, C/EBPα consists of different amino acids. Enzymes can modify proteins by attaching various methyl groups – which are small “stickers” each consisting of one carbon atom and three hydrogen atoms – to certain amino acids. This process is called methylation. In this way, they can strongly influence protein interactions. The researchers found that when one specific amino acid residue of C/EBPα is left unmethylated, it greatly accelerates the conversion process of B lymphocytes to macrophages. The methylation of this specific amino acid residue is mediated by the enzyme Carm1. Previous research has shown that Carm1-deficient mice are resistant to induced forms of acute myeloid leukaemia. This could be due to the mechanisms the researchers revealed in their study: the unmethylated version of C/EBPα is a stronger inducer of macrophage differentiation compared to its methylated counterpart. As macrophages are a non-dividing cell type, this could prevent the formation of cancer cells.
New ideas for treating leukemia
“By understanding how cell fate conversion can be accelerated or directed, we uncover new clues for cancer research”, says senior author Dr. Thomas Graf from the CRG. “For example, targeting the balance between methylated and unmethylated forms of C/EBPα can help us understand how immune cells differentiate and eventually lead to new ideas for treating certain forms of leukaemia.”
The location of the critical amino acid within C/EBPα was found when the researchers tested a mutant form of the protein called C/EBPαR35A. This mutant dramatically accelerated the speed by which B cells could be turned into macrophages. To induce a cell conversion, C/EBPα works by interacting with another transcription factor called PU.1. C/EBPαR35A was found to interact much more strongly with PU.1 than C/EBPα. This silenced B cell genes more quickly and activated macrophage genes instead.
Drugs against “confused” cells
Drugs that affect epigenetic mechanisms may indeed alter the function of transcription factors and correct cells that went astray, such as seen in cancer and leukaemia.
The methylation of C/EBPα is an example of an epigenetic mechanism. These are mechanisms which modify how the genome – the instruction manual inside every cell of the human body – is read. “Drugs that affect epigenetic mechanisms may indeed alter the function of transcription factors and correct cells that went astray, such as seen in cancer and leukaemia,” says senior author Dr. Achim Leutz, Head of the research group “Cell Differentiation and Tumorigenesis” at the Max Delbrück Center. “In this novel mechanism PU.1 is triggered by C/EBPα to switch from a B cell regulator into a macrophage regulator, an elegant ‘on-off’ mechanism that ensures the faithful formation of a mature cell type, avoiding the formation of ‘confused‘ cells often seen in blood cancers. Therefore, drugs might be found that target this mechanism to correct such defects.”
Much remains unknown about what determines the speed and directionality of cell fate decisions. The work suggests that the two processes are two sides of the same coin. For instance, how do stem cells sequentially transform into the many different types of cells in the body? Better understanding how cells change their identity and how to manipulate the process might have applications from regenerative medicine to improving the efficacy of drugs against cancer.
- About the study
-
-
The research was led by a joint collaboration between the Centre for Genomic Regulation in Barcelona, the Max Delbrück Center for Molecular Medicine in Berlin and the University of Pennsylvania in Philadelphia. The study was funded by the Spanish Ministry of Economy, Industry and Competitiveness, the Catalan Agency of Research and Universities, a 4D-Genome European Research Council Synergy grant, by funds from the Max-Delbrueck-Center, and the United States National Institute of Health.
Further information
- Graf Lab, Genome Biology, Centre for Genomic Regulation
- Researchers reveal how cells rewrite their fate – Press release by the CRG
Literature
Guillem Torcal Garcia et al (2023): Carm1-arginine methylation of the transcription factor C/EBPα regulates transdifferentiation velocity, in: eLIFE, DOI: 10.7554/eLife.83951
Download
C/EBPαR35A interacts more strongly with PU.1 than unmated C/EBPα. The images show the nuclei of cells in light blue and the interaction readout as red dots. For the wild type only 3 of 13 cells show signals, while for the mutant 7 of 8 cells are positive. Credit: Thomas Graf/Centre for Genomic Regulation
Contact
Omar Jamshed
Press Officer
Centre for Genomic Regulation
+34 623 042 388
omar.jamshed@crg.eu
Christina Anders
Editor, Communications
Max Delbrück Center
+49 30 9406-2118
presse@mdc-berlin.de
- About the CRG
-
-
The CRG is a biomedical research center located in Barcelona. Created in December 2000, the CRG is home to an interdisciplinary research team of more than four hundred scientists focused on understanding the complexity of life, from the genome to the cell and a complete organism. The CRG is a research center with a unique research model, focused on recruiting internationally-recognised leaders in the field. The CRG is a member of the Barcelona Institute of Science and Technology (BIST) and is a CERCA centre, part of the research ecosystem of the Generalitat de Catalunya.
- Max Delbrück Center
-
-
The Max Delbrück Center for Molecular Medicine in the Helmholtz Association (Max Delbrück Center) is one of the world’s leading biomedical research institutions. Max Delbrück, a Berlin native, was a Nobel laureate and one of the founders of molecular biology. At the locations in Berlin-Buch and Mitte, researchers from some 70 countries study human biology – investigating the foundations of life from its most elementary building blocks to systems-wide mechanisms. By understanding what regulates or disrupts the dynamic equilibrium of a cell, an organ, or the entire body, we can prevent diseases, diagnose them earlier, and stop their progression with tailored therapies. Patients should benefit as soon as possible from basic research discoveries. The Max Delbrück Center therefore supports spin-off creation and participates in collaborative networks. It works in close partnership with Charité – Universitätsmedizin Berlin in the jointly run Experimental and Clinical Research Center (ECRC), the Berlin Institute of Health (BIH) at Charité, and the German Center for Cardiovascular Research (DZHK). Founded in 1992, the Max Delbrück Center today employs 1,800 people and is funded 90 percent by the German federal government and 10 percent by the State of Berlin.