The last of their kind
When the bull, Sudan, his daughter, Najin, and his granddaughter, Fatu, are grazing in Kenya’s Ol Pejeta Conservancy, armed guards keep a watch on them day and night. The three northern white rhinos are the last of their kind in the world. Poachers have to be kept well at bay.
Sudan is the last bull standing.
Just guarding them, however, is not going to prevent the species from dying out because none of the animals can reproduce naturally: Sudan is old, has already had one heart attack and his low sperm count makes fertilization pretty unlikely. Najin has bad legs and would probably not be able to birth a calf. And Fatu suffers from a condition of the womb.
Undaunted, a team of researchers in Germany, Italy, Japan and the United States still wants to attempt an in vitro fertilisation – either with the help of in vitro fertilization, the method common amongst humans involving previously removed egg cells and deep-frozen sperm. However, the size of the animals and their reproductive organs present researchers with an enormous challenge. So, an alternative method may be to use modern stem cell technology to help introduce deep-frozen tissue samples from long dead northern white rhinos into the complex rescue operation and cultivate artificial gametes. If successful, the embryos would be implanted in southern white rhinos; the females of this closely related species would make suitable surrogate mothers.
Artificial fertilisation is not a routine matter
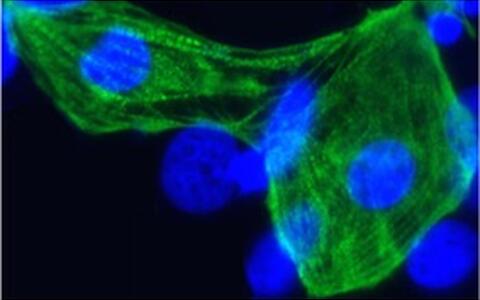
The researchers have differentiated the stem cells into cardiac cells. The protein (troponin T) appears green.
Even the first method would be a world premiere. The team supporting reproduction expert Thomas Hildebrandt from the Leibniz Institute for Zoo and Wildlife Research (IZW) have spent two years perfecting the technique they want to use to remove egg cells from the incredibly heavy animals. They are nearing their goal. At the same time, they have cooperated with partners in Italy to develop in vitro protocols so that the rhino egg cells can mature and be artificially fertilised.
The next hurdle to be taken is the period prior to implantation in the surrogate mother. Researchers have to find the right medium to enable the embryo to thrive during its first days in a petri dish. So far, in tests conducted on southern white rhinos, this has worked up to the stage of initial cell division.
Whether Najin’s and Fatu’s egg cells will suffice for these reproductive experiments is as yet unknown. Sebastian Diecke of the Max Delbrück Center for Molecular Medicine in the Helmholtz Association (MDC) is therefore working on an alternative. Together with colleagues from Japan and Munich he wants to transform the skin cells of the northern white rhino into mature eggs.
The Indiana Jones of veterinary medicine
“Thomas Hildebrandt is the Indiana Jones of veterinary medicine,” says Sebastian Diecke, head of the MDC and BIH stem cell technology platforms. “He wants to build a frozen arc of cell material from threatened animal species.” On the lookout for partners with the requisite lab experience, Hildebrandt firstly met Micha Drukker from the HelmholtzZentrum München. Drukker managed to re-program the skin cells of a northern white rhino to produce induced pluripotent stem cells (iPSCs) and called in Diecke. Together, they were able, amongst other things, to differentiate iPSCs into nerve cells and fully-functioning heart-muscle cells. To produce mature egg cells, however, is vastly more complicated.
So far, it has only been done in mice, as reported in Nature by a team around Katsuhiko Hayashi of Kyushu University in Japan in October 2016. In the lab, they managed to differentiate both embryonic stems cells and skin cells from the tips of mice tails into almost 3,200 mature egg cells. When the researchers artificially fertilised these egg cells with the normal number of chromosomes and implanted 316 embryos into surrogate mice mothers, eleven developed healthily and were born. The young mice grew normally, were fertile and did not die young. This was the result of almost a decade of painstaking work during which they had recapitulated the entire life cycle of the mouse egg cells.
Najin and Fatu are mother and daughter. They live in Kenia.
Even extinct species could be resurrected
The “egg cell engineers” are now applying this experience to the project to save the northern white rhino. Last year, Katsuhiko Hayashi spent three weeks at MDC and initiated the first experiments together with Sebastian Diecke. “To begin with, we are looking at the progenitor germ cells,” says Diecke. It is a much bigger step to produce mature egg cells and finally give birth to a rhino. “To do that, we need funding. Someone should make it a priority issue.”
Diecke is unfazed by the length of the process – what intrigues him about the project is being able to break new scientific ground while doing something very useful. “Everyone, irrespective of their previous education, understands why this is a way of implementing our stewardship of biodiversity,” says the stem cell expert. The main objective, according to Diecke, is of course to protect the animals in their natural habitat, but when a species is already on the brink, you have to do everything you can to stop them dying out altogether. “If we are successful, it might be possible at some future stage to resurrect extinct species,” he notes. The Frozen Zoo at the Beckman Center for Conservation Research in San Diego has collected habe 10.000 cell tissue samples of 1000 species so far. „One could use this ressource.“ The northern white rhino would just be the very beginning.
Long Night of the Sciences on Campus Buch
“From a skin cell to a whole animal”, June 24, 2017 from 4 pm, stand in the foyer of the Hermann-von-Helmholtz-Haus (C84); 6 pm lecture on the stage of the MDC.C foyer.