Pluripotent Stem Cells
Sebastian Diecke
Profil
The mission of the Technology Platform Pluripotent Stem Cells of the Max Delbrück Center is to support basic and translational research by facilitating all aspects of human induced pluripotent stem cell (hiPSC) technology including the derivation, genetic manipulation, differentiation, and distribution of hiPS cells.
A hallmark of our activity is precise quality control and banking activities to increase the reproducibility within the institute. Additionally, the facility provides scientists with state-of-the-art protocols, techniques and infrastructure for proper handling and manipulation of human pluripotent stem cells. Furthermore, the Technology Platform organizes hands-on training courses of standardized pluripotent stem cell culture and advanced techniques on a regular basis. At the Max Delbrück Center we are working closely together with the Transgenic and Organoid Technology Platforms to harmonize our protocols and to implement or develop new technologies.
Besides offering services of derivation, manipulation, and differentiation of induced pluripotent stem cells the technology platform constantly improves and adopts new techniques based on the requirements of the researchers in Berlin. We tidily cooperate with the Berlin institute of health (BIH) Core Unit pluripotent Stem Cells & Organoids (CUSCO) by testing different protocols or techniques as well as organizing training courses and in the past a lecture series to foster the stem cell community in Berlin.
The Core Facility was established September 2014 by funding of the Berlin Institute of health (BIH) and the German Center for Cardiovascular Disease (DZHK). To further increase the reproducibility of scientific data among different institutes, the MDC and the BIH Core Facilities for Pluripotent Stem Cells founded the PluriCore Network in Germany in 2015, sharing technical knowledge and experimental protocols. Additionally, both Core Facilities are members of international consortia like the Stem Cell COREdinates and CorEuStem - The European Network for Stem Cell Core Facilities to be part of a worldwide knowledge exchange and harmonization process of experimental procedures.
Team
Pluripotent Stem Cells
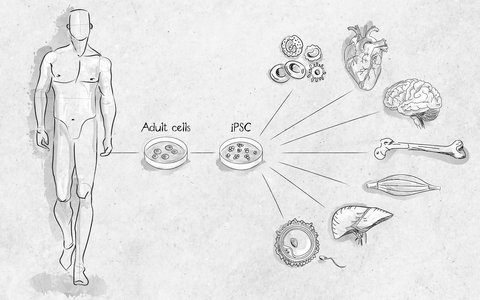
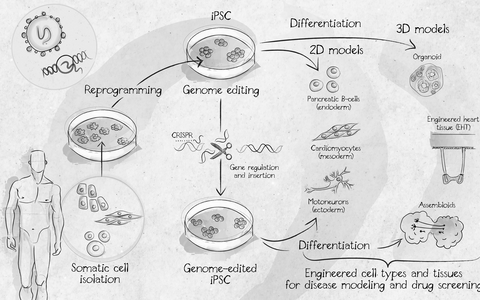
Induction of pluripotency is one of the most important scientific discoveries within the last few years. This technique allows researchers to obtain pluripotent stem cells without the controversial destruction of embryos, providing a novel and powerful tool for disease modeling and drug screening approaches.
Induced pluripotent stem cells (iPSCs) have characteristics and differentiation capacities similar to embryonic stem cell and also give rise to tissues of all three germ layers as well as whole organisms. Therefore iPSCs provide an unlimited source of proliferating cells without loss of functionality. The use of iPSCs has tremendous advantages in broad research areas of molecular mechanisms of diseases, drug testing, development of regeneration therapies and may pave the avenue towards personalized medicine.
Discovery
The description of the induction of pluripotency dramatically altered the previous dogma of cellular differentiation as a unidirectional, nonreversible developmental process, resulting in a paradigm shift in the field of developmental biology. For the breakthrough discovery Sir John B. Gurdon and Shinya Yamanaka were jointly awarded with the Nobel Prize in Physiology or Medicine 2012 "for the discovery that mature cells can be reprogrammed to become pluripotent”.
Publications
News
Service and Technology
From somatic cell isolation to application
Our Focus Areas:
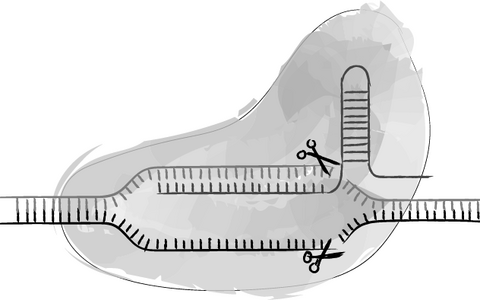
- Genome Engineering
-
-
We apply our expertise in different types of CRISPR/Cas genome engineering methods in order to facilitate flexible and affordable gene-editing services in pluripotent stem cells.
- Cardiac differentiation
-
-
Human iPSC-derived cardiomyocytes have become an invaluable component in many cardiac disease modelling, drug screening, and toxicology studies. We provide well established and standardised workflows for QC and handling of hiPSC-CMs, including functional analysis.
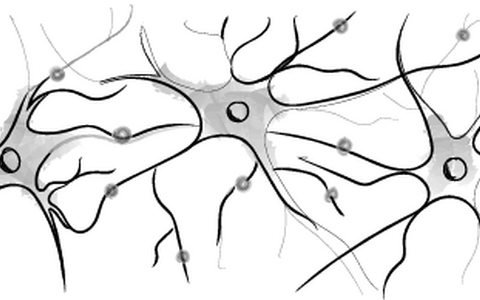
- Neuronal differentiation and Organoids
-
-
iPSCs offer unprecedented opportunities to study human brain developmental processes and disease. We provide knowledge-base and expertise for researchers interested in working with human iPSC-derived neural stem cells, functional neurons and glia cells.
- Training and Protocol development
-
-
Together with the BIH Core Facility Stem Cells we organize regular trainings on iPSC methodologies. The training is intended for researchers and technicians who are planning projects using human iPSCs and/or will start working with hiPSCs in the near future.
- Current trainings: iPSC maintenance culture
- In planning: Specialized trainings (Genome editing, Organoid differentiation)
Other Services:
- Somatic cell isolation
- Reprogramming and quality control
- Banking and provision of cells
- Differentiation into other cell types
- Project consultation
- Ressources (protocols, validated antibodies and primers,..)