The evolution of tumors
Joint press release by Charité and MDC
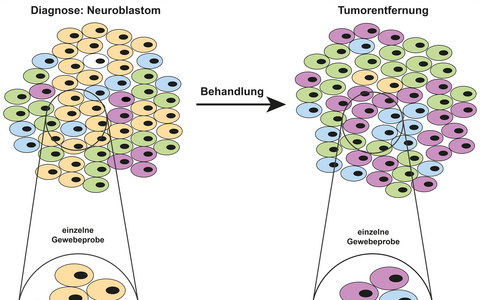
Not all cancer cells are the same: Genetically different cell subpopulations can exist in different regions in the diseased tissue. This phenomenon, known as intratumor genetic heterogeneity, is becoming an increasingly important topic in cancer research. Cellular and molecular differences within a tumor play an important role in many cancers, as these can affect diagnosis as well as the use of targeted therapies. A recent publication by Charité – Universitätsmedizin Berlin, the Max Delbrück Center for Molecular Medicine in the Helmholtz Association (MDC), and the German Cancer Consortium (DKTK) now provides evidence that the same is true of neuroblastoma, a relatively common cancer among children. These malignant solid tumors develop in the peripheral nervous system, usually originating from groups of nerve cells in the adrenal glands or along the spine and spreading from there to the abdomen.
The tumor as a mosaic: Taking a single tissue sample of a tumor composed of genetically different cells carries the risk of inaccuracy. It may not capture all the important changes in genes. Because treatment often kills most of the tumor, but often not all of the cells, further molecular testing is also needed afterwards, when the tumor is removed. Especially in the case of disease recurrence, when personalized, targeted therapy becomes very important for patients with neuroblastoma, this information is critical to the success of treatment.
“Our research shows that genetic changes typical of neuroblastoma can disappear over the course of the disease and that new ones can arise,” explains the study’s lead author Dr. Karin Schmelz from the Department of Pediatric Oncology and Hematology at Charité. “And these mutations are not evenly distributed throughout the tumor, but occur in isolated regions or even individual cells of a tumor. They form a sort of mosaic.”
Cancer is driven by evolutionary processes.
“Cancer is driven by evolutionary processes,” adds Dr. Roland Schwarz, head of the Evolutionary and Cancer Genomics Lab at the MDC and one of the study’s last authors. The cells are continuously changing their genetic composition and fighting for survival, including among themselves. They each have their own lineage trees, with some metastasizing later or becoming more difficult to treat.
Reconstructing the lineage trees of cancer cells
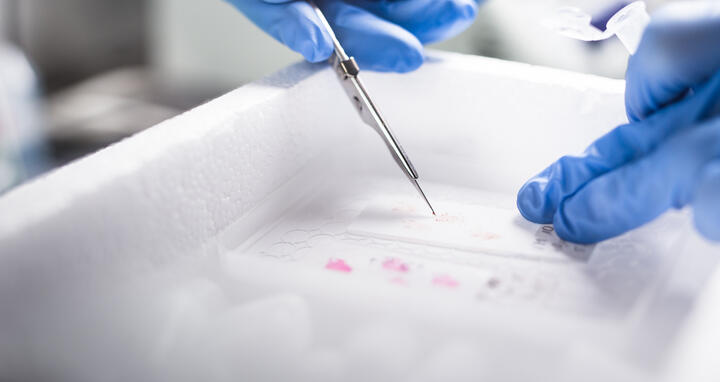
For a sensitivity analysis of genetic heterogeneity, the researchers included only regions with a high content of vital tumor cells. These were isolated with a scalpel from tissue sections of the frozen tumor material.
The research group studied a total of 140 neuroblastoma samples taken from ten children – from different tissue regions and at different time points over the course of the disease. The team evaluated each sample using a number of modern sequencing methods as well as computer-based analysis.
Detecting changes in the copy number levels of specific genes with high spatial and temporal accuracy is very complex.
The scientists were looking particularly closely at the genes ALK, MYCN and FGFR1, which play an important role in the course and treatment of the disease. They found that ALK and MYCN were not consistently present at every stage of the disease or in all tumor cells. Changes in the ALK and FGFR1 genes could be a target for therapy, especially in recurrent cancer. The scientists found that, in some patients, ALK mutations were present at the time of diagnosis but not by the time the tumor was surgically removed, while changes in the FGFR1 gene were found only in isolated tumor regions. In addition, the researchers were able to demonstrate instability in the number of gene copies of the neuroblastoma cells. In individual cases, cancer cell clones began to evolve differently from the original tumor at an early stage and spread to other organs as daughter cells, thus forming metastases.
“Detecting changes in the copy number levels of specific genes with high spatial and temporal accuracy is very complex,” explains Schwarz, a trained bioinformatician. And yet his research group – which includes lead author Dr. Matt Huska from the MDC, who now works at the Robert Koch Institute – has succeeded in developing an algorithm together with teams from the UK that can reconstruct these copy numbers with extreme precision. In 2020, Schwarz and his colleagues used this method to demonstrate continuous structural evolution in different cancer types. “We have now been able to demonstrate this for neuroblastoma, too, and to show in detail how the cancer genome changes structurally,” says Schwarz.
Initial pointers for therapy
Professor Angelika Eggert, Director of the Department of Pediatric Oncology and Hematology and the study’s last author, explains: “We now have a better understanding of how neuroblastoma cells behave. This knowledge is essential for our patients who experience disease relapse, as that is when personalized, targeted therapies often come into play. However, if the tumor shows genetic heterogeneity a molecularly targeted therapy may succeed in treating much of the diseased tissue, but not all of the cells – thus allowing the cancer to return.”
Yet she also emphasizes: “Our results are less relevant for decisions regarding the diagnosis and therapy of the first occurrence of the disease, because neuroblastoma can already be reliably diagnosed using methods that have been tried and tested for decades – such as imaging and urine tests, or even by taking a single tissue sample. And chemotherapy directed at all rapidly growing cells remains the initial treatment of choice. However, if the disease recurs, that’s when targeted treatments become particularly important. Deciding on a therapy based on a single section of tissue taken from just one site of the tumor probably does not take into account its genetic diversity. So in future, when relapse occurs, we should consider examining the tumor tissue at multiple sites using the latest sequencing technology. This would give us the most accurate information possible about the disease and enable us to make even better personalized treatment decisions.”
However, due to the technical challenges that this method still presents, the scientists are investigating other options – including the use of single-cell technologies and liquid biopsies. These novel blood tests examine the genetic material that tumors release into the blood. Taking several blood samples during the course of the disease would therefore allow for genetic changes to be detected without the need for a surgically removed tissue sample. Both methods and their clinical application are already the subject of intensive research at Charité, the Berlin Institute of Health at Charité (BIH), and the MDC.
Text: Charité
Further information
- Press release: “Cancer’s ongoing evolution”
- Department of Pediatric Oncology and Hematology (German only)
Literature
Karin Schmelz, Joern Toedling, Matt Huska et al. (2021): „Spatial and temporal intratumour heterogeneity has potential consequences for single biopsy-based neuroblastoma treatment decisions“. Nature Communications, DOI: 10.1038/s41467-021-26870-z
Download
Preparing tumor tissue for sequencing: For a sensitivity analysis of genetic heterogeneity, the researchers included only regions with a high content of vital tumor cells. These were isolated with a scalpel from tissue sections of the frozen tumor material. Photo: Charité l Linda Ambrosius
Contacts
Dr. Roland Schwarz
Head of the Evolutionary and Cancer Genomics Lab
Max Delbrück Center for Molecular Medicine in the Helmholtz Association (MDC)
+49 30 9406-3200
Roland.Schwarz@mdc-berlin.de
Dr. Karin Schmelz
Department of Pediatric Oncology and Hematology
Charité – Universitätsmedizin Berlin
+49 30 450 566 132
karin.schmelz@charite.de
Jana Schlütter
Editor, Communications Department
Max Delbrück Center for Molecular Medicine in the Helmholtz Association (MDC)
+49 30 9406-2121
jana.schluetter@mdc-berlin.de or presse@mdc-berlin.de
- Max Delbrück Center for Molecular Medicine in the Helmholtz Association (MDC)
-
The Max Delbrück Center for Molecular Medicine in the Helmholtz Association (MDC) is one of the world’s leading biomedical research institutions. Max Delbrück, a Berlin native, was a Nobel laureate and one of the founders of molecular biology. At the MDC’s locations in Berlin-Buch and Mitte, researchers from some 60 countries analyze the human system – investigating the biological foundations of life from its most elementary building blocks to systems-wide mechanisms. By understanding what regulates or disrupts the dynamic equilibrium in a cell, an organ, or the entire body, we can prevent diseases, diagnose them earlier, and stop their progression with tailored therapies. Patients should benefit as soon as possible from basic research discoveries. The MDC therefore supports spin-off creation and participates in collaborative networks. It works in close partnership with Charité – Universitätsmedizin Berlin in the jointly run Experimental and Clinical Research Center (ECRC), the Berlin Institute of Health (BIH) at Charité, and the German Center for Cardiovascular Research (DZHK). Founded in 1992, the MDC today employs 1,600 people and is funded 90 percent by the German federal government and 10 percent by the State of Berlin.