Protein controls fat metabolism
Many foods – whether it’s the mozzarella on your favorite pizza, the olive oil in salad dressing or hollandaise sauce during asparagus season – contain lots of fat. The fatty acids in these foods are among the essential nutrients that people need to survive. When someone eats more fatty acids than the body can immediately convert into energy, the extra amount is stored in tissues – often in the form of unwanted rolls of fat around the hips or stomach – and serves as a kind of reserve supply.
We have discovered that overweight people produce less EHD2 than people with normal weight.
The quantity of fatty acids transported by blood to the tissues and deposited there is determined by a wide range of factors. Researchers at the Max Delbrück Center for Molecular Medicine in the Helmholtz Association (MDC) have now identified one of these factors as the protein EHD2.
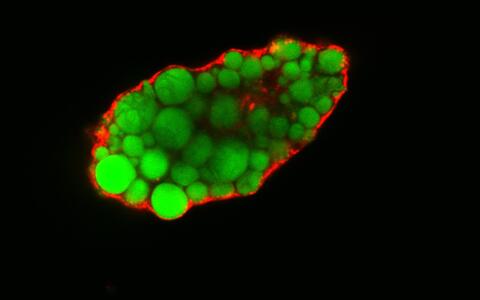
Lipidtropfen (angefärbt) in einer Fettzelle ohne EHD2.
If this protein is missing completely, fat-storing cells take up significantly more fatty acids from the cellular environment. Dr. Claudia Matthäus first observed this phenomenon in the brown adipose tissue of mice during her work at the MDC. She finds it particularly exciting that EHD2 apparently plays a key role in human fat metabolism, too. “We have discovered that overweight people produce less EHD2 than people with normal weight,” Matthäus says. It is not clear yet why this is the case. Based on these new insights, Matthäus and her colleagues, including researchers from the MDC research group led by Professor Oliver Daumke, assume that EHD2 controls a metabolic pathway that regulates fatty acid uptake in fat cells. This pathway is modified in obesity, the researchers write in the journal PNAS.
Cellular uptake of fat occurs when portions of the cell membrane pinch of
If EHD2 is not present as a stabilizer, caveolae pinch off more frequently and the cell takes up more fat.
Oliver Daumke is long acquainted with EHD2. The structural biologist has been characterizing the structure and mechanimsm of this protein for more than ten years. As a membrane protein, EHD2 resides inside muscle and fat cells. During the inward folding of the cell envelope, small flask-shaped membrane structures called caveolae are formed. These invaginations either remain on the surface of the cell membrane or they pinch off and carry foreign material – such as fatty acids – into the cell. This process is known as endocytosis, Daumke explains. The researcher assumes that the EHD2 protein assembles into ring-like structures around the neck of the membrane vessel and thus stymies the pinch-off process. Daumke is convinced: "If EHD2 is not present as a stabilizer, caveolae pinch off more frequently and the cell takes up more fat."
This is precisely what Matthäus and her colleagues examined. The researchers worked with mice in which the EHD2 gene had been switched off. Using an electron microscope, Matthäus observed that compared to normal mice, many more caveolae had become detached from the plasma membrane. She was also able to determine that fatty acid uptake was greater in cells lacking EHD2 and that the lipid droplets, which are intracellular accumulations of fat, were larger in these cells.

Live-Imaging von Mauszellen: Die einzelnen Spots, die sich in und unter der Zellmembran bewegen sind größtenteils Caveloae.
Obesity influences EHD2 production
Matthäus asked herself if she would also be able to observe an influence of EHD2 on fat metabolism in humans. So she, together with a colleague from Leipzig University, studied tissue samples from men and women with different body weight. She quickly discovered that in people who were overweight (body mass index of 25 or higher), cells produce less EHD2 than in slim people. The researcher presumes that there is a correlation between frequent membrane pinch-off and the formation of fat depots. “During obesity, we observed that the number of caveolae and their detachment from the membrane get out of sync.,” Matthäus says.
In November, Matthäus left the MDC to take a position at the National Institutes of Health in the United States. There she plans to continue her research into caveolae and fat metabolism. “There are still many unanswered questions,” Matthäus says. She is now especially interested in investigating the transport of fatty acids within the cell and the formation of lipid droplets.
Text: Christina Anders
Further information
Literatur
Matthaeus, Claudia et Al. (2020): “EHD2-mediated restriction of caveolar dynamics regulates cellular fatty acid uptake”, PNAS, DOI: 10.1073/pnas.1918415117
Downloads
Video: Live imaging of mouse cells: The spots moving in and beneath the cell membrane are mostly caveolae. © Claudia Matthäus, MDC
Photo: Lipid droplets (colored) in a fat cell that lacks EHD2. © Claudia Matthäus, MDC
Contacts
Dr. Claudia Matthäus
Postdoc researcher
The National Institutes of Health (NIH)
claudia.matthaeus@nih.gov
Professor Oliver Daumke
Head of the Research Group on Structural Biology of Membrane-Associated Processes
Max Delbrück Center for Molecular Medicine in the Helmholtz Association (MDC)
oliver.daumke@mdc-berlin.de
Christina Anders
Editor, Communications Department
Max Delbrück Center for Molecular Medicine in the Helmholtz Association (MDC)
+49 (0)30 9406 2118
christina.anders@mdc-berlin.de
- The Max Delbrück Center for Molecular Medicine (MDC)
-
The Max Delbrück Center for Molecular Medicine in the Helmholtz Association (MDC) is one of the world’s leading biomedical research institutions. Max Delbrück, a Berlin native, was a Nobel laureate and one of the founders of molecular biology. At the MDC’s locations in Berlin-Buch and Mitte, researchers from some 60 countries analyze the human system – investigating the biological foundations of life from its most elementary building blocks to systems-wide mechanisms. By understanding what regulates or disrupts the dynamic equilibrium in a cell, an organ, or the entire body, we can prevent diseases, diagnose them earlier, and stop their progression with tailored therapies. Patients should benefit as soon as possible from basic research discoveries. The MDC therefore supports spin-off creation and participates in collaborative networks. It works in close partnership with Charité – Universitätsmedizin Berlin in the jointly run Experimental and Clinical Research Center (ECRC), the Berlin Institute of Health (BIH) at Charité, and the German Center for Cardiovascular Research (DZHK). Founded in 1992, the MDC today employs 1,600 people and is funded 90 percent by the German federal government and 10 percent by the State of Berlin.