Blood vessels sprout under pressure
Prof. Gerhardt’s team is studying the many different aspects of angiogenesis – the formation of new blood vessels from existing ones. In 2014 Prof. Gerhardt moved his laboratory from London to the German capital, where he works with the Max Delbrück Center for Molecular Medicine (MDC) and the German Center for Cardiovascular Research (DZHK), holding a professorship at the Berlin Institute of Health (BIH) at the Charité. He also maintains another laboratory at the Vlaams Instituut voor Biotechnologie (VIB) in Leuven, Belgium.
The system of veins and arteries is a very complex network. At the smallest level are very fine capillaries, their wall consists of just a single layer of endothelial cells. Capillaries grow whenever blood is required for new areas of tissue – during embryonic development, wound healing or tumor growth, for example.
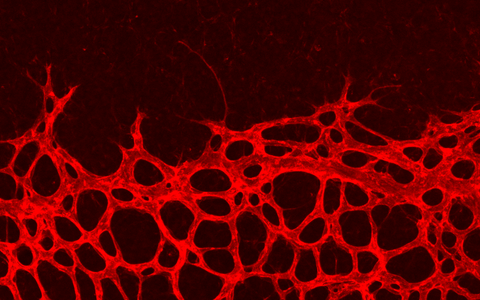
The formation of capillary loops, magnified 20x: New capillaries are forming in the retina of a mouse at the periphery (top) in arc-like structures.
During “sprouting angiogenesis”, new cells grow from the wall of existing nearby capillaries to make a new capillary loop. Holger Gerhardt explains: “At first these capillary sprouts are just a series of endothelial cells without a lumen” – that is, without the internal space through which the blood will later flow. Until now, scientists did not know how the lumen forms, says Gerhardt. To illustrate this point, his PhD student Véronique Gebala produces video images from the publication of another working group: “The temporal resolution is not good enough – you can see cavities, but not how they formed.”
A new paper published in Nature Cell Biology by Gebala, Gerhardt and their colleagues provide the first highly-resolved account of the process of lumen formation. Using cutting-edge technology–a spinning-disk confocal microscope that provides high spatial and temporal resolution–the researchers studied zebrafish embryos in which the endothelial cell membranes had been genetically labeled with a fluorescing protein. Several hours after fertilization of the egg, Gebala tracked how new capillaries form in the embryos: the blood squeezes into the endothelial cell and, as it does so, the membrane of the new lumen grows. This is a completely new concept in angiogenesis that had not been previously described. The process is not confined to fish: Véronique Gebala also observed the intermediate stages of the process in mouse retina preparations.
When the blood pressure is sufficiently high, it overcomes the barrier of the cell cytoskeleton and forms a membrane invagination that spreads further and further into the cell. The new lumen keeps changing shape and sometimes collapses. If the blood pressure is reduced by means of drugs or if the afferent blood vessel is severed with a fine laser beam, blood vessel formation is halted completely, demonstrating that blood pressure is indeed the driving force behind lumen formation.
As the invagination grows into the cell body, the cell actively controls its progression. It pushes back smaller side branches and small bubbles by building actin fibers and then contracting them with the help of myosin filaments, just like a cellular muscle. This way the new capillary lumen only grows at the tip. This was the second important insight, as Holger Gerhardt explains: “We started off thinking that these blebs develop inward into the cavity. In fact the topology is exactly the opposite, but nobody before us had seen it.” To check whether actomyosin filaments really do control the lumen growth, the researchers genetically switched off the myosin molecules in the cells. They found that the invaginations were no longer retracted and that this resulted in malformed lumens that ballooned uncontrollably.
This property of the lumen is relevant to various diseases, says Gerhardt: “If blood vessel formation depends so heavily on the hydrodynamics of the blood, what does this mean for physiological blood pressure?” He explains that we know that unstable blood vessels are rapidly removed and that there is no constant reconstruction of blood vessels in the body. He adds that perhaps in certain diseases this might happen. For example, retinal blood vessels are destroyed in the eyes of diabetics, especially at sites affected by strong fluctuation in blood pressure. Diabetes is one of the most common causes of sight loss in adults.
Angiogenesis is also of major importance in cancer. Growing tumors attempt to satisfy their ravenous hunger for energy and oxygen and let blood vessels sprout. “Blood vessels in tumors are not normal. They are leaky and often unable to control their diameter,” says Gebala. Now the researchers are keen to find out how important the newly discovered mechanism of blood vessel is: “The next logical step is to study pathological situations,” says Gebala. The project still has much potential for further insights.
This is where the working group benefits from its affiliation to the BIH. The new institute facilitates exchange between basic science and clinical research on patients. Holger Gerhardt feels that there is great potential here, which was ultimately what drew him to Berlin. His involvement in a number of projects is proving worthwhile for the laboratory – for example, he was able to buy the confocal microscope with grants from the DZHK. And the fact that Gerhardt has labs at various locations and in various subject areas boosts scientific creativity: “It means that the pools of ideas from different work environments can come together, and that is very fruitful. Of course it is very demanding, but also rewarding.”
Highlight Reference:
Véronique Gebala, Russell Collins, Ilse Geudens, Li-Kun Phng, Holger Gerhardt (2016): "Blood flow drives lumen formation by inverse membrane blebbing during angiogenesis in vivo." Nature Cell Biology. doi:10.1038/ncb3320