Nephrology and Inflammatory Vascular Diseases
Profile
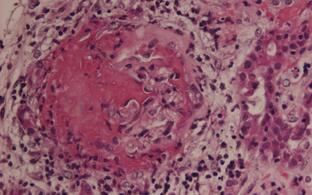
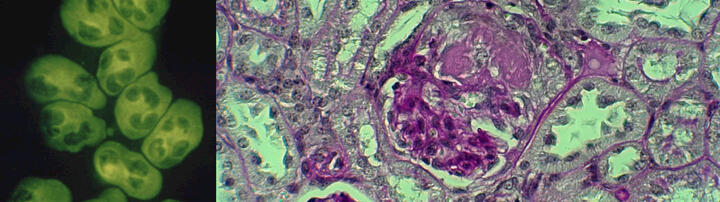
We are interested in systemic autoimmune vasculitides, particular those featuring anti-neutrophil cytoplasmic autoantibodies (ANCA). We care for an ANCA vasculitis patient cohort at the Charite clinics and explore molecular mechanisms by which ANCA-activated innate immune cells damage the endothelium leading to necrotizing vasculitis in our laboratory at the ECRC.
Team
Research
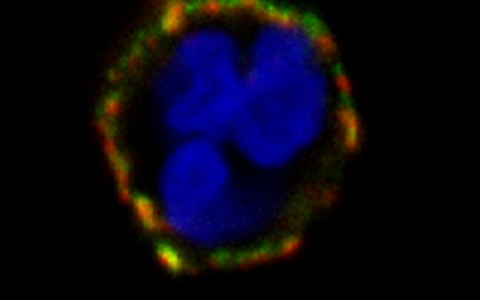
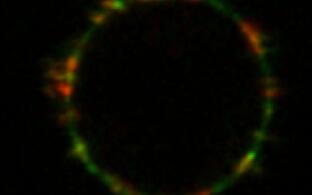
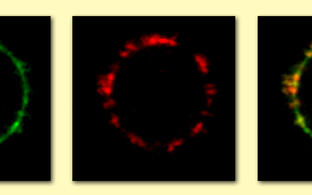
An initial step in the inflammatory cascade is binding of ANCA to their autoantigens on the cell surface of neutrophils and monocytes. Proteinase 3 (PR3) and myeloperoxidase (MPO) are the major ANCA target antigens. In contrast to MPO which is found on all neutrophils, PR3 shows a bimodal surface pattern with low- and high-expressing neutrophil populations. This bimodal pattern results from neutrophil subset-restricted expression of the PR3 receptor CD177 that supports high PR3 amounts on the neutrophil surface. We study genetic and epigenetic mechanisms that are responsible for this clinically relevant bimodal CD177-mediated mPR3 pattern. Moreover, we explore strategies to disrupt the CD177-PR3 complex to reduce PR3-ANCA binding and neutrophil activation.
CD177 provides an example for neutrophil heterogeneity. We study causes and functional consequences of this phenomenon with implications above-and-beyond ANCA vasculitis. For example, CD177 ligands other than PR3 may exist. We will search for such ligands using biological samples from patients with inflammatory diseases.
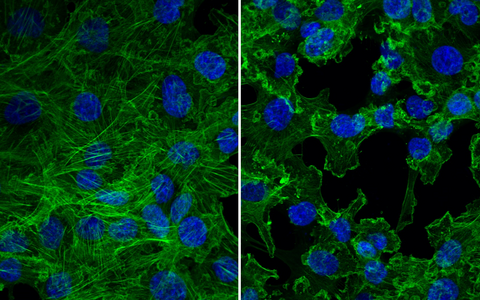
ANCA bind to and activate the neutrophil and monocyte. Study ANCA-induced responses in these innate immune cells that contribute to vascular inflammation. Moreover, we study neutrophil and monocyte interactions and how these interactions modify inflammation.