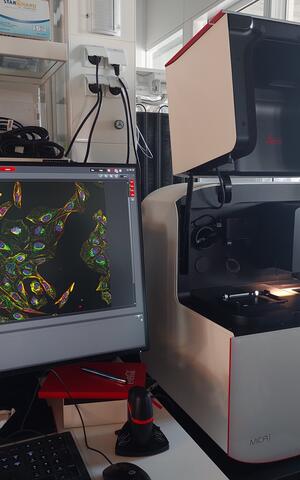
Systems Biology Imaging
Robert P. Zinzen
About
We offer consultations and collaborations to help create research workflows including custom building experimental parts, preparing samples, imaging & other light based techniques, and designing bespoke image analysis pipelines.
Overview
Our infrastructure incorporates a range of light-based biotechnology techniques, providing images across volume, time and spatial resolution scales. We have a well equipped lab space and workshop for preparing samples and experimental parts, a number of image and data processing tools, and can offer expertise to help with experimental design, light based measurements and image analysis solutions.
In addition to supporting the work of other researchers, we are also involved with a number of collaborations and projects of our own, and seek to foster interactions with industry partners to bring emerging imaging technologies to the campus.
Current Projects
We are working to adapt existing or developing novel technology including:
- Small molecule and genetically encoded biosensors
- Experimental multiplexing techniques
- Advanced image analysis tools
to quantitatively study intracellular signal transduction and synaptic transmission. We aim at contributing to a better understand how information is processed at subcellular, cellular, and networks scales and how this processing is altered in neurological disorders.
Platform Video Tour
The Systems Biology Imaging team members present a tour around the equipment and workspaces available in the platform.
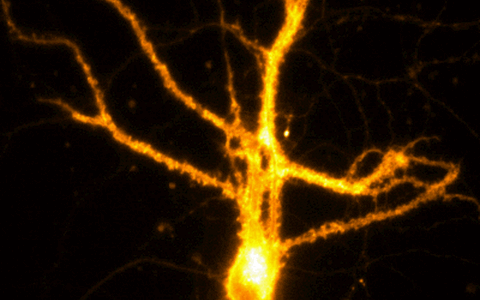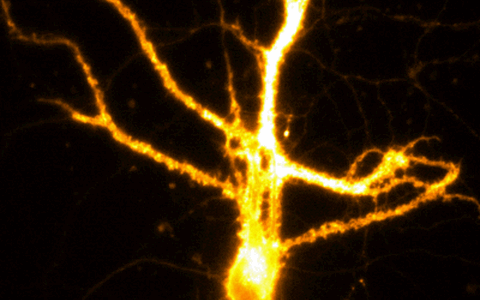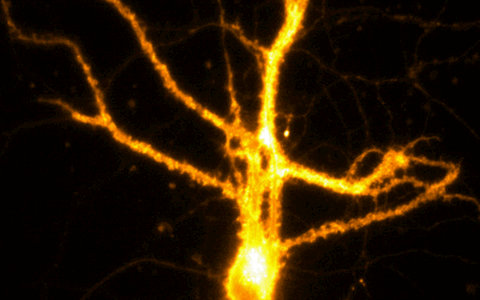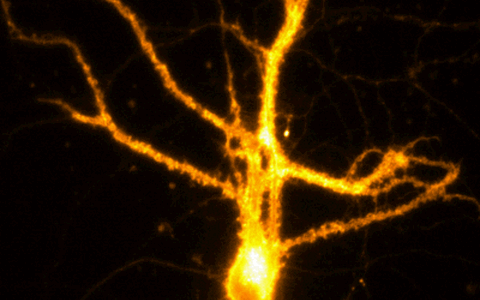
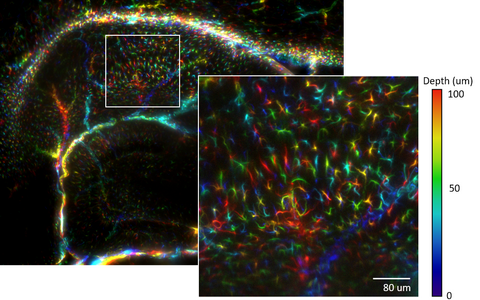
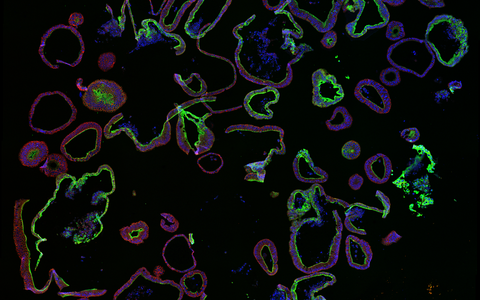
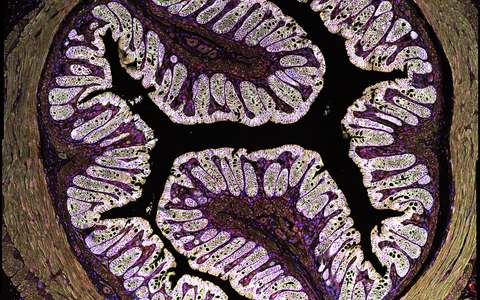
Example Image Gallery
Events
Upcoming Events
Microscopy Club | monthly
Previous Events
11th Berlin Summer Meeting and BIMSB Opening Symposium | 25-27 October 2018
Leica workshop on Multiphoton Confocal and Advanced Live Cell Imaging | 19-22 September 2017
MDC Technology Training in Light Microscopy / Zeiss workshop | 11-13 July 2017
10th Berlin Summer Meeting "Imaging Gene Regulation from DNA to RNA to protein” | 8-10 June 2017
EU-Life Course Biological Image Reconstruction and Analysis | 14-18 November 2016
Team
Group Leader
Dr. Robert P. Zinzen
101: Berlin Institute for Medical Systems Biology: 1.07
Technical Assistant
Agnieszka Klementowicz
101: Berlin Institute for Medical Systems Biology: 2.50.1
Agnieszka.Klementowicz@mdc-berlin.de
Scientists
Dr. Peran Hayes
101: Berlin Institute for Medical Systems Biology: 2.50.1
davidperan.hayes@mdc-berlin.de
Student Assistant
Setenay Şimal Delioğlu
101: Berlin Institute for Medical Systems Biology: 2.50.1
SetenaySimal.Delioglu@mdc-berlin.de
Former Members
Dr. Andrew Woehler
Dr. Nikita Vladimirov
Dr. Anna Oliveras Martinez
Dr. Zohreh Farsi
Dr. Marie Walde
Elisabeth Fritsch
Giulia Forcina
Linda Drewicke
Ecem Çakmak
Equipment
Confocal Microscopes
- Leica SP8 Falcon (SMD)
- Versatile confocal with a wide range of functionalities
-
Good for:
- High resolution, 3D imaging of thicker samples.
- Multi-channel imaging: customizable excitation & detection wavelengths to minimize crosstalk
- Customizable image format & area eg for resolution optimization, FRAP, photoactivation...
- Spectral scanning, FLIM & Fluorescence lifetime gating
- User-friendly software
Specs:
- White Light Laser coupled with an Acousto-Optic Beam Splitter to allow tunable illumination within a wavelength range of 470-670nm and a bandlet width of <3nm
- 405nm near-UV diode laser
- Leica DMi8 Microscope body
- Prism detection system to facilitate tunable detection spectra control across an emission spectrum of 380-800nm
- 4 Hybrid detectors & 1 PMT detector
- Transmitted light detector
- Chamber for CO2 and temperature control for live imaging
- Options to control scan area, image format & aspect ratio and image rotation
- λΛ-Scan Spectral scanning functions
- Fluorescence Lifetime Imaging module and detection lifetime gating
- Navigator module for large sample tile-scanning and automated multi-point imaging
- Leica Lightning confocal deconvolution software
- Leica SP8 STELLARIS
- Confocal microscope with super-resolution capabilities
-
Good for:
- High resolution, 3D imaging of thicker samples.
- Super-resolution imaging (STED)
- Multi-channel imaging: customizable excitation & detection wavelengths to minimize crosstalk
- Customizable image format & area eg for resolution optimization, FRAP, photoactivation...
- Spectral scanning, FLIM & Fluorescence lifetime gating
- User-friendly software
Specs:
- White Light Laser coupled with an Acousto-Optic Beam Splitter to allow tunable illumination within a wavelength range of 470-670nm and a bandlet width of <3nm
- 405nm near-UV diode laser
- 590nm STED laser
- Prism detection system to facilitate tunable detection spectra control across an emission spectrum of 380-800nm
- 2 Hybrid detectors & 2PMT detectors
- Chamber for CO2 and temperature control for live imaging
- Options to control scan area, image format & aspect ratio and image rotation
- λΛ-Scan Spectral scanning functions
- Detector lifetime gating
- Lightning confocal deconvolution software
- Andor Dragonfly 200 Spinning Disk
- Spinning disk confocal for fast 3D imaging
-
Good for:
- High speed imaging to capture fast dynamic samples or image large volumes
- Confocal optical sectioning to image thick samples in 3D at high resolution
- 3D SMLM: resolution to 10-20 nm
- Large field of view
- Borealis® Uniform Illumination that provides stable light throughput, uniform illumination, and extended wavelength range.
- Active blanking, laser illumination synchronised with camera exposure.
Specs:
- Sona Back-Illuminated sCMOS Camera
- 1400 x 1400 pixels,
- 11 μm camera chip pixel size
- 15.4 x 15.4 mm image area
- ZL41 Cell sCMOS Camera
- 2048 x 2048 pixels
- 6.5 μm camera chip pixel size
- 13.3 x 13.3 mm image area
- Leica DMi8 Microscope body
- Two pinhole size options: 25 μm or 40 μm)
- 7 excitation laser lines: 405, 445, 488, 514, 561, 694 & 637nm
- Emission filters: 450/50, 480/40, 525/50, 540/30, 600/50, 647/57, 700/75
- Max confocal scan rate: 400 scans per second
- Lateral resolution Diffraction limited in 16.6 x 14.0 mm (21.7 mm diagonal rectangular FOV, 19 mm diagonal square FOV)
- Leica Mica
- Widefield-Confocal "Microhub" with the benefits of both plus incubation.
Good for:
- Uniting widefield and confocal imaging with sample protection and incubated environment.
- Generates fast overviews with widefield imaging, later high resolution images of a region of interest can be acquired using confocal.
- Limited to 4 different labels due to use of Leica's FluoSync spectral un-mixing approach.
- Intelligent automation and AI-supported analysis.
- Sequential and simultaneous acquisiton possible.
Specs:
- Possible simultaneous Detection channels: 4 with FluoSync™ fluorophore separation for widefield and confocal
- 4 LEDs for widefield imaging: 365 nm, 470 nm, 555 nm, 625 nm
- 4 diode lasers for confocal imaging: 405 nm, 488 nm, 561 nm, 638 nm
- 4 Cameras for widefield imaging: 5 megapixel CMOS
- 4 detectors for confocal imaging: HyD FS Detector
- Objectives: PL FLUOTAR 1.6x/0.05 (air), PL FLUOTAR 10x/0.32 (air), CS2 objective 20x/0.75 (air), CS2 objective 63X/1.2 (Automatic water)
- Reflection-based Adaptive Focus Control (AFC)
- CO2 and temperature control for live imaging
Widefield Microscopes
- Leica THUNDER Live Cell Imager
- Fast, user-friendly and multi-functional widefield system
-
Good for:
- Fast imaging of large sample areas
- A good option for thin samples
- Multi-position acquisitions and stitched tile scans
- Colour camera, eg for histology, H&E staining etc.
- Multi-channel imaging with 8 LEDs
- User-friendly software
Specs:
- Leica LED8 Light Engine has 8 illumination LEDs with wavelength peaks at 395, 438, 475, 511, 555, 575, 635 and 730 nm
- Compatible filter sets appropriate for most labels within the visible spectrum.
- Leica DMi8 microscope body
- Leica DFC 9000GT sCMOS monochrome fluorescence camera:
- sensor size: 13.3mm x 13.3mm
- number of pixels: 2048 x 2048
- sensor pixel size: 6.5µm x 6.5µm
- min exposure time: <12 ms
- Bit depth: 12 bit / 16 bit
- Max quantum efficitency: ~82% @ 580nm
- Leica FLEXACAM C1 colour CMOS camera:
- sensor size: 4.55 x 6.17mm (1/2.3")
- number of pixels: 4000 x 3000 (12MP)
- sensor pixel size: 1.55µm x 1.55µm
- exposure time: 100µs - 120ms
- colour depth: 3 x 8 bit
- Navigator module for large sample tile-scanning and automated multi-point imaging
- Leica Thunder widefield deconvolution software
To test/check possible microscope configurations, detection efficiency and potential crosstalk for your specific fluorophores, please follow this FPbase link.
- Nikon/Visitron iLas2 TIRF
- Widefield system with TIRF, FRAP, FLIP and localised photoactivation capabilities
-
Good for:
- TIRF microscopy, which can image a single thin slice (<100nm thick) at the sample-coverslip interface with high resolution & signal to noise ratio
- FRAP & FLIP techniques to study molecular dynamics
- Locally controlled illumination and photoactivation (eg for glutamate uncaging)
- An alternative to the THUNDER for simple widefield imaging (brightfield, phase & polarisation contrast, DIC and epi-fluorescence)
Specs:
- Four Coherent Obis laser illumination lines: 405, 488, 561 and 640nm
- Visitron Systems Laser combiner
- Gataca Systems iLas2 laser control platform for 360° spinning, uniform field TIRF with simultaneous FRAP, FLIP & photoactivation
- Nikon Eclipse Ti microscope body
- Visitron Systems VisiView Microscope control software
- Photometrics Prime 95B back-illuminated sCMOS camera
- Nikon / MMI LCM
- Multi-channel widefield fluorescent imaging system with laser microdissection and single cell selection capabilities.
Good for:
- Single Cell microdissection via laser ablation
- Single cell micro-capillary aspiration & deposition
- Multi-channel widefield imaging
- Temperature & Co2 environmental control
Specs:
- Lumencor SPECTRA III (L) Light Engine with 6 LED light sources with wavelength peaks at 390, 440, 475, 510, 555 & 575nm and 2 Lasers at 635 & 747nm
- Prior OptiScan III motorised stage control system
- Nikon Eclipse Ti 2 microscope body
- Photometrics Prime 95B 22mm Camera
- MMI CellEctor capillary cell sorting tool. Capable of manual and automated micro-capillary based single cell recognition, aspiration and deposition.
- MMI CellCut laser microdissection tool. Designed for manual cell identification, cutting and extraction.
- MMI CellTools software
- OKOlab Temperature & CO2 environmental control setup.
Light-Sheet Microscopes
- Zeiss Z.1
- Dual light-sheet microscope for fast, gentle, 3D volume imaging
Good for:
- Light-sheet microscope can image fluorescently labeled living samples.
- Can image model organisms, tissues and cells over days with virtually no phototoxicity or bleaching.
- Can collect Z-stacks from the desired angle of viewing or from a number of different angles, in multiple positions and with different zoom factors
- Combining cylindrical lens optics and laser scanning to generate the illumination light sheet.
Specs:
- Choice of 4 laser lines: 405 nm, 488 nm, 561 nm, 638 nm with controllable power output.
- Transmission LED for brightfield sample positioning and overview
- Detection Module “Standard”, ICX 285 CCD, 1388 × 1036 pixels
- Equipped with 5x (0.16 NA), 10x (0.5 NA) & 20x (1.0 NA) detection objectives and 5x (0.1 NA) & 10x (0.2 NA) detection objectives
- Configurable for refractive index mounting of 1.33 (water) with 5x, 10 or 20x detection and 1.42-1.48 (glycerol) for 5x or 20x detection.
- CO2 -Module and heating device humidity. Temperature stability: ± 0.1 °C
- Field of View: 60 μm to 2.8 mm
- Embedded specimen size from < 1 µm to 5 mm
- Light sheet thickness 2 μm – approx. 14 μm (depending on sample, at 488 nm)
- mesoSPIM
- Open source, in-house built system for meso-scale volume imaging
Good for:
- Unlike other light sheet microscopes, MesoSPIM can produce volumetric images of centimeter sized cleared samples like whole mouse brains, spinal cords with excellent image quality over large fields-of-view.
- Multi-position acquisitions and stitched tile scans.
- Has about 50x50x100 mm travel range.
- Offers 360° vertical-axis sample rotation.
- Compatible with different clearing techniques, both active and passive, eg CLARITY, X-CLARITY, CUBIC-X, BABB, iDISCO, MASH...
- Reduced shadowing artifacts due to pivot-scanning.
- Axially scanned light-sheet microscope can achieve roughly isotropic resolution of ~6µm in X-Y-Z across the FOV.
Specs:
- Four Cobolt CW Lasers: 405 nm, 488 nm, 561 nm and 636 nm
- Olympus MVX10 Zoom Microscope Body
- Magnification range: 0.63x - 6.3x.
- Hamamatsu ORCA-FLASH4.0 V3 Digital CMOS Camera C13440
- Effective no. of pixels: 2048×2048
- Effective area: 13.312 mm×13.312 mm
- Camera chip pixel size: 6.5 μm×6.5 μm (image pixel size depends on magnification)
- Quantum efficiency: 82 % (peak QE: @560 nm)
Maker Space
- 3D Printer Ultimaker2 + Extended
-
- FDM/FFF based
- Construction volume: 223 x 223 x 305 mm
- Single extruder
- Filament diameter: 2.85mm
- Nozzle diameter: 0.25mm, 0.4mm, 0.6mm and 0.8mm
- Max. nozzle temperature; 260 C°
- max. print bed temperature 100 C°
- Print quality: 0.015mm
- 3D Printer FormLabs Form2
Auto-heats to 35 C°
- Self-heating Resin Tank
- Laser: EN 60825-1:2007 certified, Class 1 Laser Product, 405nm violet laser, 250mW laser
- Stereolithography (SLA)
- Peel Mechanism: Sliding Peel Process with wiper
- Automated Resin Fill System
- Construction volume: 145 × 145 × 175 mm
- Layer Thickness (Axis Resolution): 25, 50, 100 microns
- Laser Spot Size (FWHM): 140 microns
- 3D Printer Keyence AGILISTA-3200W
-
- Print material: AR-M2 (transparent printing material), AR-H1 (heat-resistant printing material), AR-G1L (Silicone Rubber [Low Hardness]), AR-G1H (Silicone Rubber [High Hardness])
- Support material: AR-S1 (water-soluble support material)
- Installation space: 297 × 210 × 200 mm (A4 size × 200 mm)
- Resolution: 635×400dpi
- Layer thickness in high resolution: AR-M2: 15µm
- Layer thickness in normal resolution, AR-M2: 20µm, AR-H1: 20µm, AR-G1L/H: 30µm
- Milling/Drilling machine:Holzmann BF 16V
-
- Bandsaw: Proxxon MBS 240/E
-
- Other
- Jigsaw: Bosch PST 18 LI Cordless
- Hot air gun: Einhell TH-HA 2000/1
- Various hand tools, clamps etc.
Other
- Tissue clearing support
- LifeCanvas EasyGel tissue-gel hybridisation system
- LifeCanvas SmartClear II pro multi purpose clearing system
- LifeCanvas Smart Label active immunostaining device
- High Power Expanded Plasma Cleaner
PDC-002-CE
- Used for nanoscale surface cleaning, surface activation and surface chemistry
- Electronics
- Soldering station: Toolcraft ST-50 A
- Oscilloscope: Rigol DS1054Z
- Waveform generator
- Multimeter
- Optics
- Spectrometer
- Power meter
- Refractometer
- Various optics/optomechanics parts
- Microfluidics
- Soft lithography infrastucture
- Microfluidics controllers
Publications
News
Training & Resources
Training
Theoretical and hands on training required for all systems (est. 2 hrs).
Booking
Go to https://mdc.science-it.ch/ to register for our booking system. After registering, go to the browse tab, then resources, search for the instrument you are interested in using (i.e. SP8 SMD), click on the blue arrow under the device picture to request access. The platform will get an email and contact you to schedule a training session. After which we will grant you access and you can book the system anytime you like.
Resources
Refractive Index Matching
It is important to choose your imaging objective such that its refractive index matches as closely as possible that of your imaging mounting media
| Water | 1.33 |
|---|---|
| Silicone | 1.406 |
| Glycerol | 1.47 |
| Oil | 1.518 |
| 75% Glycerol | 1.44 | |
|---|---|---|
| Vectashield | 1.457 | |
| Mowiol | 1.49 | |
| ProLong Gold | Fresh mounting Cured for 1 day Cured for 4 days | 1.39 1.40 1.44 |
Useful Links