Experimental and Clinical Research Center
Lindenberger Weg 80
13125 Berlin
We pursue a “next generation staging” in order to better understand the dynamic changes during conventional chemotherapy, targeted therapies, and immunotherapies. For this purpose, we longitudinally and comprehensively monitor dynamic modifications of the immune system, the tumor and the tumor microenvironment enforced by the chosen treatment.
Aims are the gain of critical individualized information for tailored treatment approaches (combination strategies, patient selection, novel targets) and the development of novel strategies to restore the immune competence after treatment.
Prof. Dr. Il-Kang Na
il-kang@charite.de
Tel: +49 30 450- 540155/ -653598
Fax: +49 30 450- 7540155
Dr. Marco Frentsch
marco.frentsch@charite.de
Tel: +49 30 450-540173
Dr. Elisa Ciraolo
Dr. Phillip Schiele
Dr. Maren Schmiester
maren.schmiester@charite.de
Tel: +49 30 450-539513
Dr. Friedrich Wittenbecher
friedrich.wittenbecher@charite.de
Tel: +49 30 450-539513
Dr. Eva Tranter
Stanislav Rosnev
Anna Walter
Charlotte Junkuhn
charlotte.junkuhn@charite.de
Tel: +49 30 539 535
Pia Wolf
Josefine Russ
josefine.russ@charite.de
Tel: +49 30 450-540458
Julia Vahldick (TA)
David Busch
Adoptive T cell transfer (ATT) of antigen-specific T cells can be a remarkably efficient cancer therapy, but durable complete responses are rarely achieved in the treatment of solid tumors. Recent advances have widened the spectrum of therapeutics to be used in combination with ATT in order to overcome inhibitory mechanisms presented by solid tumors and their microenvironment. Their evaluation would greatly benefit from an in vivo monitoring tool allowing the detection of functional parameters of transferred T cells.
We generated a transgenic mouse line BLITC (bioluminescence imaging of T cells) expressing an NFAT-dependent Click-beetle luciferase and a constitutive Renilla luciferase facilitating longitudinal in vivo studies on T cell activation and migration in various mouse models. We could demonstrate its suitability in two independent tumor models employing tumor-antigen mono-specific CD4+ and CD8+ T cells. Our BLI data demonstrated rapid tumor infiltration but suggested transient T cell activation to be causative for the failure to reject solid tumors, further emphasizing the importance of accessory treatments for ATT.
BLITC reporter in the H-Y tumor model. Female albino RagKO mice were challenged with 104 MB49 cells 12 days before ATT. Tumor-bearing mice were sublethally irradiated (2 × 1.5 Gy within 4 hours) before transfer of no T cells (controls not shown), 5 × 104, or 5 × 105 ex vivo ML-BLITC CD4+ T cells as indicated. A, T-cell migration and activation were monitored in vivo via Rluc and NFAT-CBR BLI and one representative mouse is shown for selected time points. Red circles: ROIs used for calculation of TIL flux. B, Left, The mean luminescence intensities of Rluc and NFAT-CBR for TILs as exemplified by the ROIs shown for day 14. †, Mice sacrificed because of tumor size; Right, tumor growth curves for all experimental groups are shown on top (data representative of 3 independent experiments; n = 4 mice). All graphs display mean + SEM.
In addition, the transfer of T cells involves the risk of adverse effects due to on-target off-tumor toxicity. Using a clinically relevant minor histocompatibility antigen (MiHA) H-Y tumor model, we monitored alloreactivity upon single MiHA mismatch in female -> male transfers in order to investigate on-target off-tumor toxicity. Using our bioluminescent reporter system we are currently testing treatment strategies for optimized T cell mediated cancer therapy.
Graft-versus-Host disease (GVHD) is a survival-limiting complication after allogeneic hematopoietic stem cell transplantation (allo-HSCT). Major target organs are the skin, the gut and the liver. We could show that donor alloreactive T cells also damage the bone marrow leading to osteoblast loss and hampered B cell reconstitution.
Patients with late onset of B-cell reconstitution display increased numbers of bone marrow-infiltrating T cells associated with reduced numbers of osteoblasts. Histological sections of BM trephines obtained 3-4 weeks after allo-HSCT were analysed for the number of CD3+ T cells and osteoblasts. Graphs show representative examples for the staining of formalin-fixed sections from patients with early (upper graphs) or late (lower graphs) onset of B-cell reconstitution. Osteoblasts are indicated with arrows, T cells are stained by anti-CD3 antibody (brown) and are marked with triangles.
Memory cell deficiency after HSCT leads to a high susceptibility to fatal infections and therefore strategies to overcome this long-lasting immunodeficiency are required. Recently, it has been demonstrated that the BM is a reservoir for memory T cells, which are antigen-experienced immune cells crucial for immunity against a broad variety of pathogens. Only a few studies indicate memory B cells as persisting in BM and other lymphoid organs.
Since BM-resident memory T cells have been characterized with distinct functional features compared with their circulating counterparts, particularly exhibiting a more resting state (Okhrimenko et al. 2014), we have performed a comprehensive comparative analysis using paired BM and PB samples. We found human BM memory B cells to express less HLA-DR and CXCR3 but increased Fas-Ligand compared to their PB counterparts. Upon re-activation, BM memory B cells exhibited a more resting phenotype. Transcriptome and protein analyses revealed a higher amount of α-defensin expression in BM memory B cells. BM-serum comprised α-defensin RNA- and protein-rich exosomes. Co-culture of sorted PB lymphocytes with BM-serum or with CD45+CD15+ cells from PB and BM, classical α-defensin expressers, induced α-defensinhigh memory cells. BM mononuclear cells were significantly more bactericidal than PB mononuclear cells.
RNA of switched and non-switched memB from paired human peripheral mononuclear cells (PBMC) and bone marrow mononuclear cells (BMMC) samples of three different donors (D1-D3) was used for microarray analysis. The gene expression pattern is illustrated for indicated differentially expressed genes as heatmap based on the LIMMA algorithm.
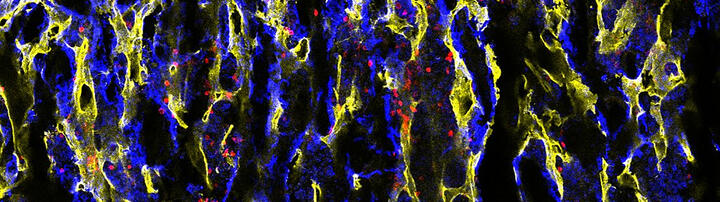
In a collaborative research project with the research group of Olaf Penack (Charité) and Georg Duda (BCRT), we aim to characterize the memory T and B cell reconstitution after HSCT and to elucidate the role of the BM microenvironment for the memory T and B cell survival in the BM. Furthermore, we will test immunotherapeutical and pharmacological strategies to improve the restore the BM niche, in order to facilitate accelerated seeding and maintenance of memory T and B cells into the BM and to provide thereby long-term immunity after HSCT. For this purpose, we apply 3D immunofluorescence imaging (see profile image by Sarah Mertlitz).
Long-term survival after allogeneic hematopoietic stem cell transplantation (allo-HSCT) requires a fully reconstituted immune system, which is hampered by lymphoid organ damage associated with conditioning therapy, graft-versus-host disease, and immunosuppression. We are interested to better understand the immune defects occurring in allo-HSCT.
Thymic graft-versus-host disease (tGVHD) can contribute to profound T cell deficiency and repertoire restriction after allo-HSCT. Using clinically relevant murine allo-BMT models, we could show that even minimal numbers of donor alloreactive T cells, which caused mild nonlethal systemic graft-versus-host disease, were sufficient to damage the thymus, delay T lineage reconstitution, and compromise donor peripheral T cell function. Furthermore, donor alloreactive T cells used the cognate proteins FasL and TNF-related apoptosis-inducing ligand (TRAIL) (but not TNF or perforin) to mediate tGVHD, thereby damaging thymic stromal cells, cytoarchitecture, and function. Strategies that interfere with Fas/FasL and TRAIL/DR5 interactions may therefore represent a means to attenuate tGVHD and improve T cell reconstitution in allo-HSCT reciepients.
Rabbit antithymocyte globulin-Genzyme™ is used to prevent GVHD after-HSCT. Since the effects of rabbit antithymocyte globulin-Genzyme™ on thymic function have not been well-studied yet, we analyzed the kinetics of conventional and regulatory T cells in adult patients treated or not treated with rabbit antithymocyte globulin-Genzyme™ during the first 6 months after allo-HSCT. Patients treated with rabbit antithymocyte globulin-Genzyme™ had almost undetectable levels of recent thymic emigrants (CD45RA(+)CD31(+)) of both conventional and regulatory CD4 T cells throughout the 6 months after allogeneic hematopoietic stem cell transplantation whereas CD4(+)CD45RA-memory T cells were less affected, but their levels were also significantly lower than in patients not treated with rabbit antithymocyte globulin-Genzyme™. These data support thymus-protective therapies in patients treated with rabbit antithymocyte globulin-Genzyme™ could benefit from thymus-protective therapies.
In an attempt to identify the mechanisms contributing to sustained low memory B cell numbers after allo-HSCT, we performed reconstitution kinetics and functional assays. Upon CD40/TLR-9-dependent activation, B cells underwent significantly increased apoptosis paralleled by an aberrant up-regulation of Fas-L on activated T cells and Fas on resting B cells. Significantly increased B cell apoptosis was also observed after CD40/BCR and CD40/BCR/TLR-9-dependent activation. Follow-up studies evaluating effectiveness of revaccinations on the cellular level and addressing the long-term sequelae of B cell defects after transplantation are ongoing.
Increased CD40/BCR and CD40/BCR/TLR-9-induced B cell apoptosis in allo-HSCT patients. PBMCs were stimulated with anti-IgM/CD40L (upper graphs) or with anti-IgM/CD40L/CpG (lower graphs). Shown are the percentages of propidium iodide (PI)−AnnexinV+ early apoptotic, PI+AnnexinV+ late apoptotic, and PI+AnnexinV− necrotic B cells. Lines indicate median values. Significant differences between HC and patients are given. **P ≤ .001.
Currently, treatment decisions are typically made based on initial tumor and patient characteristics “at diagnosis”. However, dynamic therapy-induced remodeling in cancer patients remains largely neglected, although these changes may profoundly interfere with drug efficacy and tumor immune control.
Hypothesizing that a better molecular and functional characterization of tumor and host properties in response to therapy will improve patient outcome by guiding informed cancer precision medicine, we propose a comprehensive screening of tumor, host and tumor/host interface parameters at various time-points during therapy. Starting with patients suffering from diffuse large B-cell lymphoma (DLBCL), we are establishing a comprehensive monitoring algorithm.