Autoantibodies in COVID-19: not always harmful?
In the summer of 2020, a discovery by a French research group led by immunologist Jean-Laurent Casanova caused a sensation. His team had found antibodies in the blood of critically ill COVID-19 patients that were directed against the signaling molecule type 1 interferon. The body produces interferon to protect itself from viruses, but when autoantibodies bind to interferon, they render it ineffective – thus leaving the patient defenseless.
“This had led many immunologists to begin looking for further autoantibodies in the blood of COVID-19 patients,” says Dr. Kathrin de la Rosa, who holds the Johanna Quandt Professorship for Translational Immune Mechanisms at the Berlin Institute of Health at Charité (BIH) and heads a lab at the Max Delbrück Center. “And they’ve found quite a few: Researchers have so far identified a total of 17 antibodies that target the body’s own structures, including the ACE2 protein, which serves as a receptor for the virus.”
Autoantibodies recognize a broad range of targets
Mikhail Lebedin and Kathrin de la Rosa in the lab.
De la Rosa and her team also examined the blood of COVID-19 patients treated at Charité. They, too, found autoantibodies against the ACE2 protein, against interferon alpha and against other body proteins. “Interestingly, there was not a clear relationship between soluble ACE2 and ACE2-binding autoantibodies. The temporal appearance of the autoantibodies was also an argument against an ACE2-directed immune response,” reports Mikhail Lebedin, a PhD student in de la Rosa’s lab and lead author of the present paper. “However, we did find a correlation between the amounts of various autoantibodies, which got our wheels turning.”
The antibody specialists wondered how it was possible that a COVID-19 infection could produce different antibodies in equal amounts. Could it be one and the same antibodies with the ability to bind to different body proteins? Were they so-called multispecific antibodies?
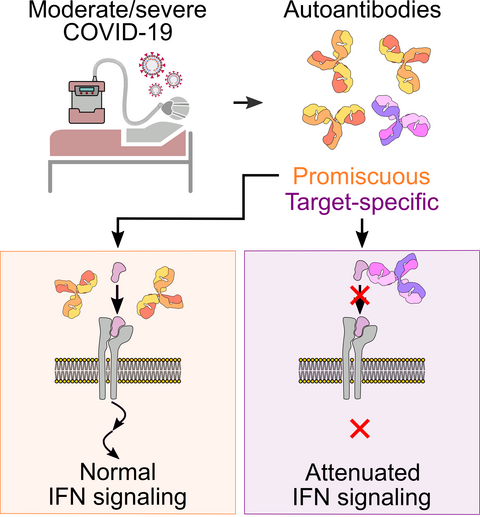
The scientists then analyzed the reactivity of the antibodies present in the blood of COVID-19 patients. The vast majority of the samples exhibited polyreactive autoimmunity, as the antibodies recognized a wide variety of proteins to a similar extent. The autoantibodies were directed at a specific target in only a few samples. “In terms of COVID-19, the question was now whether multispecific antibodies could influence disease progression, or whether target-directed autoantibodies have the exclusive ability to do this,” says de la Rosa. “Promiscuous antibodies also occur in other infectious diseases such as glandular fever and HIV.”
Promiscuous autoantibodies have no impact on interferon signaling pathway
The scientists then evaluated the functionality of the antibodies they had found. In the petri dish, they mixed healthy blood cells with the patients’ autoantibodies and observed whether this had any effect on the growth or behavior of the cells. “The cells did not respond at all to the presence of the polyreactive antibodies,” reports Lebedin. “It was only when we added targeted monospecific antibodies to the cells that their behavior changed.”
De la Rosa does not want to cast doubt on the importance of autoantibodies in general. “It is certain that autoantibodies against type 1 interferon in particular contribute to severe disease progression,” she says. However, when it comes to diagnostics and therapeutics, further tests should be done to verify that antibodies are actually present that very specifically recognize a certain body protein. It could be that all we are dealing with is an essentially harmless representative of the multispecific autoantibodies.”
Text: BIH
Further information
Literature
Mikhail Lebedin et al (2023): „Discriminating promiscuous from target-specific autoantibodies in COVID-19“. European Journal of Immunology, DOI: 10.1002/eji.202250210
Picture to download
Kathrin de la Rosa in the lab.
Photo: Pablo Castagnola, Max Delbrück Center
Contacts
Prof. Dr. Kathrin de la Rosa
Head of the Lab “Cancer & Immunology / Immune Mechanisms and Human Antibodies”
Max Delbrück Center
Kathrin.delaRosa@mdc-berlin.de
Dr. Stefanie Seltmann
Head of Communications
Berlin Institute of Health at Charité (BIH)
+49 (0) 30 450 543019
stefanie.seltmann@bih-charite.de
Jana Schlütter
Editor, Communications
Max Delbrück Center
+49 (0)30 9406 2121
jana.schluetter@mdc-berlin.de oder presse@mdc-berlin.de
- BIH Johanna Quandt Professorships
-
-
Stiftung Charité and the BIH have jointly launched the BIH Johanna Quandt Professorships (temporary W2 professorships with a genuine tenure track). The novel professorship scheme targets specifically female scientists in order to provide an impetus for the promotion of equal opportunities in the life sciences. The professorships are filled through an international recruitment process and include a binding option for permanent tenure as a lifetime professorship (genuine tenure track). In addition, the professorships are open to all topics, offering the applicants the opportunity to develop the orientation of their professorships themselves, also beyond the usual biomedical disciplines, and to have an innovative impact on the BIH’s translational mission. Along with the three Johanna Quandt Professors selected in 2017, a total of six BIH Johanna Quandt Professorships now enrich the life sciences in Berlin.
- Max Delbrück Center
-
-
The Max Delbrück Center for Molecular Medicine in the Helmholtz Association (Max Delbrück Center) is one of the world’s leading biomedical research institutions. Max Delbrück, a Berlin native, was a Nobel laureate and one of the founders of molecular biology. At the Center’s locations in Berlin-Buch and Mitte, researchers from some 70 countries analyze the human system – investigating the biological foundations of life from its most elementary building blocks to systems-wide mechanisms. By understanding what regulates or disrupts the dynamic equilibrium in a cell, an organ, or the entire body, we can prevent diseases, diagnose them earlier, and stop their progression with tailored therapies. Patients should benefit as soon as possible from basic research discoveries. The Max Delbrück Center therefore supports spin-off creation and participates in collaborative networks. It works in close partnership with Charité – Universitätsmedizin Berlin in the jointly run Experimental and Clinical Research Center (ECRC), as well as with the Berlin Institute of Health (BIH) at Charité and the German Center for Cardiovascular Research (DZHK). Founded in 1992, the Max Delbrück Center today employs 1,800 people and is funded 90 percent by the German federal government and 10 percent by the State of Berlin.