Disease genes help early brain development
While some sufferers have no or only mild symptoms, others have to live with severe deformities – even if the two patients are related to each other and we can therefore assume that the disease is caused by the same gene mutation.
Holoprosencephaly (HPE) affects around one to four in every 1,000 unborn babies, and occurs when the cerebral hemispheres of the forebrain fail to divide or only partially divide. It is the most common malformation of the forebrain in humans and is associated with facial disfigurements, such as cleft lip and cleft palate or eyes that are very close together – even to the extent that the two eyeballs merge. The majority of fetuses affected die while still in the womb.
The exact causes of HPE are not yet fully understood. In addition to environmental pollutants and illness of the expectant mother, genetic factors can play a role – including mutations in the genes of the so-called Sonic Hedgehog (SHH) signaling pathway. This pathway controls the embryonic development of organs and the nervous system. Gene defects and a resulting loss of function of LRP2, an SHH co-receptor, result in brain defects that manifest themselves very differently. “We wanted to know why the severity of this disease varies so much,” says Dr. Annette Hammes, head of the Molecular Pathways in Cortical Development Lab at the Max Delbrück Center for Molecular Medicine in the Helmholtz Association (MDC). “While some sufferers have no or only mild symptoms, others have to live with severe deformities – even if the two patients are related to each other and we can therefore assume that the disease is caused by the same gene mutation.”
Disease genes restore the SHH pathway
Researchers have long assumed that there are genes that positively influence this malformation or even prevent it altogether. The Hammes Lab team has now identified two new candidates: “ULK4 and PTTG1, also known as securin,” says co-lead author Dr. Nora Mecklenburg, who was a postdoctoral researcher with Hammes at the time of the study. ULK4 is a gene that has so far been associated with schizophrenia and bipolar disorder, while PTTG1 is mainly researched in connection with cancer. In the journal Development, the scientists explain how these proteins can restore an impaired SHH pathway. Nine years of work went into the study, which the journal classifies as a “research highlight.”
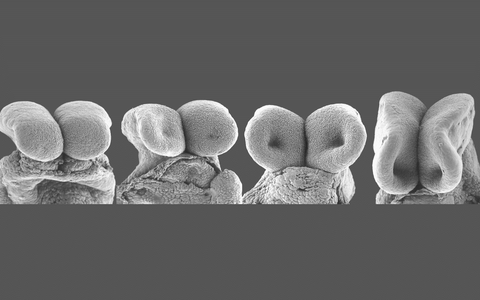
Morphological changes of the mouse neural folds during neural tube formation.
The extent of the differences even between the wild types of the two mouse strains at this early stage of embryonic development really surprised us.
But the results were also partly down to chance. Hammes and her team had been studying mice with LRP2 mutations for many years, together with the MDC lab led by Professor Thomas Willnow. “We know that LRP2 influences the formation of the neural tube in early embryogenesis, and it is out of this that the nervous system later develops,” explains the neuroscientist. Without LRP2, the SHH pathway is not sufficiently activated and malformations of the neural tube occur at a very early stage of pregnancy – often resulting in miscarriage. When the scientists crossed the LRP2 mutants of the commonly used black-coated mouse strain (known as “Black 6” for short) with another mouse strain with white coats, the result was very surprising: The white-coated offspring did not show any malformations of the brain or face – despite having a mutation in the SHH co-receptor LRP2. The team concluded that there must be as-yet-unknown factors that influence the SHH pathway, and set out to find them.
RNA analysis with high-throughput sequencing
To do this, Mecklenburg first bred the different mouse strains and examined the animals with regard to their disease characteristics, signaling pathways and genetic makeup. Together with her co-lead authors Franziska Witte, then a doctoral student in Professor Norbert Hübner’s MDC lab, and Izabela Kowalczyk, a doctoral student with Hammes, she sequenced and analyzed the RNA of embryonic cells of the different strains using high-throughput methods. The three scientists discovered that, despite having a similar genome sequence, the cells have completely different transcriptomes – meaning the genes are read very differently. “The extent of the differences even between the wild types of the two mouse strains at this early stage of embryonic development really surprised us,” says Mecklenburg.
More ULK4 and PTTG1 are produced in the LRP2 mutants with the white mouse ancestors. They compensate for the missing LRP2, restore a sufficiently strong SHH pathway, and prevent the malformations from occurring.
The researchers conducted further studies and found that, in the white-coated LRP2 mutants, as well as in the wild types of this strain, certain genes, including ULK4 and PTTG1, are strongly upregulated compared to the Black 6 mice. To see if this affects the SHH pathway, they introduced the genes into cells lacking LRP2 function. “We were able to see that they significantly boost the Sonic Hedgehog signaling pathway,” says Kowalczyk. Their conclusion: “More ULK4 and PTTG1 are produced in the LRP2 mutants with the white mouse ancestors. They compensate for the missing LRP2, restore a sufficiently strong SHH pathway, and prevent the malformations from occurring.” This finding casts the disease genes ULK4 and PTTG1 in a completely new light: While high levels of expression can trigger diseases in the adult organism, they can actually positively influence the development of an embryo. The scientists were also able to pinpoint the location from which these factors amplify the SHH pathway – the antenna-like projections of the neuroepithelial cells, which are the cells that line the inside of the neural tube.
Decoding – and maybe even preventing – genetic diseases
“The fact that we have identified these candidate genes that modulate the SHH pathway in mice takes our knowledge of holoprosencephaly and other genetic diseases one step further,” says Hammes. “With this knowledge, we may even find a way to prevent them.” But there is still a long way to go until then, and the next step for her team is to explore what role the newly discovered SHH pathway modifiers play – not only during embryonic development, but also in the adult brain. The scientists have already located PTTG1 in the cytoskeleton of neurons – a protein network in the cytoplasm that gives the cells stability. The team is currently investigating what role the gene plays in this location.
Text: Jana Ehrhardt-Joswig
Further Information
Literature
Mecklenburg, Nora et al. “Identification of disease-relevant modulators of the SHH pathway in the developing brain.” Development. https://doi.org/10.1242/dev.199307
Downloads
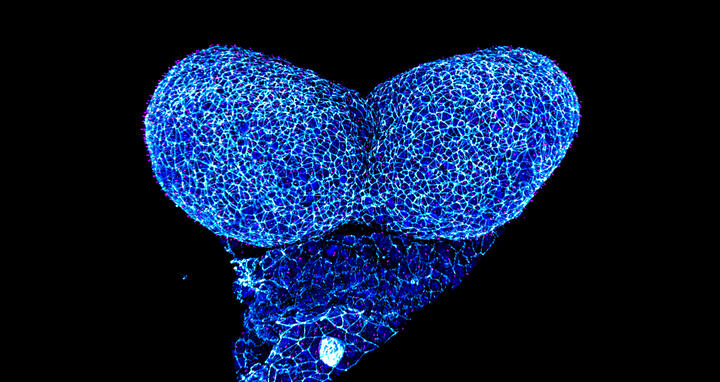
Anterior neural folds of a mouse embryo imaged at Advanced Light Microscopy technology platform at MDC. Credit: Hammes Lab, MDC
Morphological changes of the mouse neural folds during neural tube formation. Credit: Hammes Lab, MDC
Contacts
Dr. Annette Hammes
Molecular Pathways in Cortical Development Lab
Max Delbrück Center for Molecular Medicine in the Helmholtz Association (MDC)
Tel.: +49-(0)30-9406-3825
hammes@mdc-berlin.de
Jana Ehrhardt-Joswig
Editor, Communications Department
Max Delbrück Center for Molecular Medicine in the Helmholtz Association (MDC)
Tel.: +49-(0)30-9406-2118
jana.ehrhardt-joswig@mdc-berlin.de or presse@mdc-berlin.de
- Max Delbrück Center for Molecular Medicine in the Helmholtz Association (MDC)
-
-
The Max Delbrück Center for Molecular Medicine in the Helmholtz Association (MDC) is one of the world’s leading biomedical research institutions. Max Delbrück, a Berlin native, was a Nobel laureate and one of the founders of molecular biology. At the MDC’s locations in Berlin-Buch and Mitte, researchers from some 60 countries analyze the human system – investigating the biological foundations of life from its most elementary building blocks to systems-wide mechanisms. By understanding what regulates or disrupts the dynamic equilibrium in a cell, an organ, or the entire body, we can prevent diseases, diagnose them earlier, and stop their progression with tailored therapies. Patients should benefit as soon as possible from basic research discoveries. The MDC therefore supports spin-off creation and participates in collaborative networks. It works in close partnership with Charité – Universitätsmedizin Berlin in the jointly run Experimental and Clinical Research Center (ECRC ), the Berlin Institute of Health (BIH) at Charité, and the German Center for Cardiovascular Research (DZHK). Founded in 1992, the MDC today employs 1,600 people and is funded 90 percent by the German federal government and 10 percent by the State of Berlin.