Lighting up tumor cells
When the cell’s state changes, certain transcription factors become active and bind to the corresponding target genes – and to our reporter. And then the cell lights up.
The Curt Meyer Memorial Prize of the Berlin Cancer Society, which is endowed with €10,000, commemorates Senate Councillor Dr. Curt Meyer (1891–1984), who as a physician and health policymaker was committed throughout his life to educating people about cancer and preventing and fighting the deadly disease. This year’s prize goes to Matthias Jürgen Schmitt of the Max Delbrück Center for Molecular Medicine (MDC). The award ceremony will take place on October 1, 2021, at the annual meeting of the German Society for Hematology and Medical Oncology in Berlin. “Although the prize was presented to me, it recognizes the success of our entire team. And we’re tremendously thrilled that the jury has honored our work – work into which we have expended a lot of energy and even some frustration,” says Matthias Schmitt. “We’re also proud that our young lab was recognized, especially considering that most previous award winners were much further along in their scientific careers.”
Resistance is crux of problem
Matthias Schmitt is a PhD student in Dr. Gaetano Gargiulo’s Molecular Oncology Lab at the MDC. Together with Yuliia Dramaretska and Juan Carlos Company Nevado he has developed molecular reporters to study how glioblastomas manage to become resistant to any therapy – and how this may be prevented. These resistance mechanisms must be elucidated before new therapeutic options for this form of cancer can be developed. “In the past 20 years, not a single new effective drug has come onto the market. And that’s despite the fact that no other type of tumor is so well molecular biologically characterized as glioblastoma,” Schmitt stresses.
Matthias-Jürgen Schmitt in the lab
Um neue Therapieoptionen für diese Krebsform entwickeln zu können, müssen diese Resistenzmechanismen aufgeklärt werden. „In den vergangenen 20 Jahren kam kein einziges neues wirksames Medikament auf den Markt. Und das, obwohl keine andere Tumorart molekularbiologisch so gut charakterisiert ist“, betont Matthias Schmitt.
The problem is that most studies to date have focused on the biopsy and molecular characterization of the entire tumor. Yet the composition of a glioblastoma changes at the single-cell level over time, particularly when the tumor returns after an initially successful therapy. In this process the tumor cells often transition to the most aggressive subtype. Molecular reporters can now make this “identity change” visible.
Light signals from the cell
Molecular reporters are synthetic copies of DNA sequences that regulate the activity of those genes that initiate or stop cell transformation. “We have virtually combined the complete ‘regulome’ of these signature genes into a small piece of DNA and fused it to a fluorescent protein,” the molecular biologist explains. “When the cell’s state changes, certain transcription factors become active and bind to the corresponding target genes – and to our reporter. And then the cell lights up.”
The cells of origin for glioblastoma likely develop from neural stem cells and from glial cells, the supporting tissue of the brain. They strongly infiltrate into surrounding tissue during this process. In Germany alone, about 4,800 people are newly diagnosed with this very aggressive cancer every year. The diagnosis is still a death sentence. That’s because even if the standard therapy – surgical removal of the tumor followed by radiation and chemotherapy – is initially successful, the tumor invariably returns, and patients die within a few months. Drugs that are used successfully to treat other forms of cancer do not reach this tumor at all because they cannot cross the blood-brain barrier.
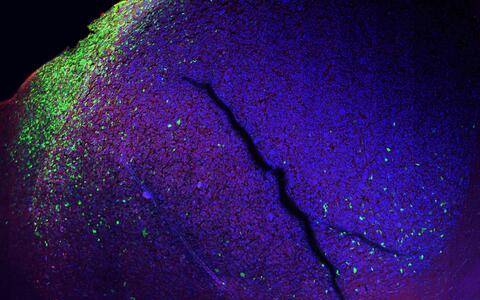
Molecular reporter exposes collaborators
Previously only suspected, now made visible thanks to the molecular reporter: When human tumor cells and brain cells of a mouse meet, the tumor cells begin to change (green).
New single-cell technologies have revealed just how heterogeneous a glioblastoma is. “We were able to see that there are many different cell types at different stages, and that a one-size-fits-all therapy is not possible,” Schmitt explains. As if it weren’t complicated enough that diverse genetic, epigenetic and transcriptional factors play a role in resistance development, cells in the immediate tumor microenvironment also get involved in the process.
We now know that if you attack one cell type with chemotherapy, the composition of the tumor changes to another cell type.
With the help of molecular reporters, Schmitt and his colleagues were able to visualize how cells of the innate immune system actually “protect” the tumor cells instead of fighting them. They help the tumor cells change their identity to the most aggressive subtype of glioblastoma, the subtype that is maximally resistant to therapies. “We now know that if you attack one cell type with chemotherapy, the composition of the tumor changes to another cell type,” says Schmitt. “One possible approach would be to use this evasive maneuver to reduce the number of cell states and force the tumor to transition into the least aggressive type.”
The researchers can now track in real time how individual tumor cells respond to specific therapies. The team wants to find out if and how it is possible to stop immune cells from protecting tumor cells. The concept of fluorescent reporters also happens to be applicable not only to tumors, but also to many other biological questions
Co-award winner: Dr. Laura Schmalbrock of Charité
Matthias Jürgen Schmitt shares this year’s Curt Meyer Memorial Prize with Dr. Laura Schmalbrock from the Department of Hematology, Oncology and Tumor Immunology at Charité – Universitätsmedizin Berlin. She is being honored for her work on acute myeloid leukemia (AML), the most common form of acute leukemia in adults. Her research has examined why some patients do not respond to an established method of therapy involving tyrosine kinase inhibitors in combination with chemotherapy or why they suffer a relapse of the disease.
Text: Catarina Pietschmann
Further information
Literature
Matthias Jürgen Schmitt, Carlos Company, Yuliia Dramaretska et al (2020): Phenotypic mapping of pathological crosstalk between glioblastoma and innate immune cells by synthetic genetic tracing, Cancer Discovery, DOI: 10.1158/2159-8290.CD-20-0219
Contacts
Matthias Jürgen Schmitt
Molecular Oncology Lab
Max Delbrück Center for Molecular Medicine in the Helmholtz Association (MDC)
Tel.: +49 30 9406-3863
matthias.schmitt@mdc-berlin.de
Jana Ehrhardt-Joswig
Editor Communications Department
Max Delbrück Center for Molecular Medicine in the Helmholtz Association (MDC)
Tel.: +49-(0)30-9406-2118
jana.ehrhardt-joswig@mdc-berlin.de oder presse@mdc-berlin.de
- Max Delbrück Center for Molecular Medicine in the Helmholtz Association (MDC)
-
The Max Delbrück Center for Molecular Medicine in the Helmholtz Association (MDC) is one of the world’s leading biomedical research institutions. Max Delbrück, a Berlin native, was a Nobel laureate and one of the founders of molecular biology. At the MDC’s locations in Berlin-Buch and Mitte, researchers from some 60 countries analyze the human system – investigating the biological foundations of life from its most elementary building blocks to systems-wide mechanisms. By understanding what regulates or disrupts the dynamic equilibrium in a cell, an organ, or the entire body, we can prevent diseases, diagnose them earlier, and stop their progression with tailored therapies. Patients should benefit as soon as possible from basic research discoveries. The MDC therefore supports spin-off creation and participates in collaborative networks. It works in close partnership with Charité – Universitätsmedizin Berlin in the jointly run Experimental and Clinical Research Center (ECRC), the Berlin Institute of Health (BIH) at Charité, and the German Center for Cardiovascular Research (DZHK). Founded in 1992, the MDC today employs 1,600 people and is funded 90 percent by the German federal government and 10 percent by the State of Berlin.