Profile
We are particularly interested in the ubiquitin proteasome system (UPS). We showed earlier that muscle RING finger (MuRF) 1 and MuRF3 are important for maintaining cardiac and skeletal muscle structure and function. We are currently identifying and characterizing the regulation and function of MuRF proteins in myocytes. We identified the stress responsive serine/threonine kinase protein kinase D1 (PKD1) as an important regulator of pathological cardiac remodeling and fiber type specification in skeletal muscle. We investigated PKD1-dependent control of the class IIa histone deacetylase/myocyte enhancer factor-2 axis in chronic cardiac stress situations, and work on non-transcriptional functions of PKD1 in heart and skeletal muscle.
Team
Group Leader
Prof. Dr. med. Jens Fielitz
jens.fielitz@charite.de
Phone: +49 (30) 450 540424 /- 421
Fax: +49 (30) 450 540928
Scientists
Dr. rer. nat. Dörte Lodka
doerte.lodka@charite.de
Phone: +49 (30) 450 540578 /- 428
Dr. rer. nat. Melanie Kny
melanie.kny@charite.de
Phone: +49 (30) 450 540578 /- 428
Technical Assistants
Sibylle Schmidt
sibylle.schmidt@charite.de
Phone: +49 (30) 450 540 424 /- 422
PhD students
Alexander Hahn
alexander.hahn@charite.de
Phone: +49 (30) 450 540578 /- 428
Cristina Pablo
cristina.pablo-tortola@charite.de
Phone: +49 (30) 450 540 428
Medical doctoral candidates
Katharina Busch
katharina.busch@charite.de
Phone: +49 (30) 450 540 428
Julius Grunow
julius.grunow@charite.de
Phone: +49 (30) 450 540 428
Sebastian Wundersitz
sebastian.wundersitz@charite.de
Phone: +49 (30) 450 540 428
Lukas Zanders
lukas.zanders@charite.de
Phone: +49 (30) 450 540 428
Master students
Sarah Bützow
Falk Fischer
falk-ike.fischer@charite.de
Phone: +49 (30) 450 540 428
Research
Research Projects
The ubiquitin proteasome system (UPS) is largely responsible for the degradation of long-lived proteins, such as components of the contractile apparatus of striated muscle cells. The UPS is activated during cardiac hypertrophy, heart failure and skeletal muscle atrophy leading to degradation of structural and contractile proteins, most notably myosin heavy chain, resulting in a reduction of cardiac and muscle function. Within the UPS E3 ubiquitin ligases mediate substrate specificity and are the rate limiting enzymes.
We investigate the regulation and function of the muscle-specific E3 ubiquitin ligases Muscle RING-finger (MuRF) 1, 2 and 3 in myocyte remodeling. We showed that muscle RING finger (MuRF) 1, 2 and MuRF3 are important for maintaining cardiac and skeletal muscle structure and function. Combined deletion of MuRF1 and MuRF3, and MuRF2 and MuRF3 resulted in protein aggregate myopathy of the heart and skeletal muscle. Nevertheless, the function of MuRF proteins in muscle cells is not well understood. Therefore, we are currently identifying and characterizing the regulation and function of MuRF proteins in greater detail.
Protein homeostasis is also impaired in muscle of critically ill patients. We reported an imbalanced protein homeostasis with decreased synthesis and increased degradation of muscular proteins in muscle of patients with Intensive Care Unit (ICU)-acquired weakness (ICUAW; a major comorbidity of critically ill patients). Although, inflammation is thought to be involved in the pathogenesis of ICUAW the precise disease mechanisms are not well understood.
We identified the stress responsive serine/threonine kinase protein kinase D1 (PKD1) as an important regulator of pathological cardiac remodeling and Ang II-induced muscle wasting. We described PKD1-dependent control of the class IIa histone deacetylase 5/transcription factor EB (TFEB) axis in muscle atrophy, and work on non-transcriptional functions of PKD1 in heart and skeletal muscle.
- Function of PKD1 in pathological cardiac remodeling
-
Function of the stress-dependent kinase Protein Kinase D1 (PKD1) in pathological cardiac remodeling
-
The adult heart responds to biomechanical stress and neurohormonal signaling by hypertrophic growth, accompanied by fibrosis, and activation of a fetal gene program leading to a diminished cardiac function. Class II histone deacetylases (HDACs) are negative regulators of pathological cardiac remodeling via the myocyte enhancer factor-2 (MEF2) transcription factor, an activator of heart disease. Protein kinase D1 (PKD1) is a stress-responsive kinase that phosphorylates class II HDACs, resulting in their dissociation from MEF2 with subsequent activation of MEF2 target genes. Cardiac PKD1 is activated in response to arterial hypertension, pressure overload, and chronic neurohormonal activation.
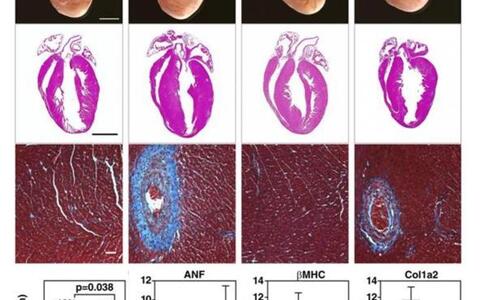
We generated mice with a conditional PKD1-null allele (PKD1 cKO). Mice with cardiomyocyte-specific deletion of PKD1 showed diminished cardiac hypertrophy, interstitial and perivascular fibrosis, and fetal gene activation as well as improved cardiac function in response to chronic pressure overload or chronic angiotensin II (Ang II; Figure 1) and adrenergic signaling. These findings demonstrate that PKD1 in cardiomyocytes plays a key role in mediating stress-dependent remodeling and reprogramming of gene expression in the adult heart. However, in addition to transcriptional regulation PKD1 regulates myocyte contractility by phosphorylating contractile proteins. In further projects, we focus on non-transcriptional functions of PKD1 in acute cardiac stress response.
The effects of cardiomyocyte specific PKD1 elimination (cKO) in angiotensin II (Ang II) induced cardiac remodeling are shown. After Ang II treatment, PKD1 mutant mice (cKO) displayed less cardiac hypertrophy and a reduction in perivascular and interstitial fibrosis (hematoxylin and eosin and Masson's trichrome stainings; top panel). Gen expression of cardiac stress markers (i.e. Atrial natriuretic factor (ANF)), the fetal gene program (i.e. ß-myosin heavy chain (β-MHC)), and fibrosis (i.e. procollagen, type I, α2 (Col1α2)) was reduced in PKD1 mutant mice (bottom panel). Our data show that deletion of PKD1 in cardiomyocytes protects the heart from pathological cardiac remodelling.
- Function of PKD1 in Ang II-induced muscle wasting
-
Function of the stress-dependent kinase Protein Kinase D1 (PKD1) in angiotensin II (Ang II) induced muscle atrophy
-
Skeletal muscle wasting with accompanying cachexia is a life threatening complication in congestive heart failure. The molecular mechanisms are imperfectly understood, although an activated renin–angiotensin aldosterone system has been implicated. Angiotensin (Ang) II induces skeletal muscle atrophy in part by increased muscle-enriched E3 ubiquitin ligase muscle RING-finger-1 (MuRF1/Trim63) expression, which may involve protein kinase D1 (PKD1).
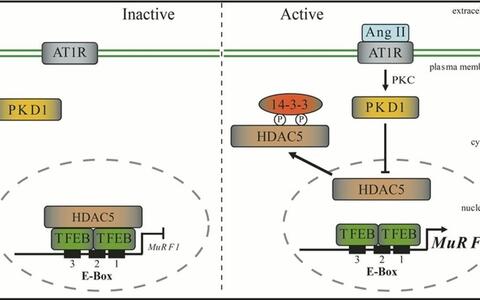
In a recent study we elucidated the molecular mechanism of Ang II–induced skeletal muscle wasting. In a cDNA expression screen we identified the lysosomal hydrolase-coordinating transcription factor EB (TFEB) as novel regulator of the human MuRF1/Trim63 promoter. TFEB played a key role in regulating Ang II–induced skeletal muscle atrophy by transcriptional control of MuRF1/Trim63 via conserved E-box elements. Inhibiting TFEB with small interfering RNA prevented Ang II–induced MuRF1/Trim63 expression and muscle atrophy. The histone deacetylase-5 (HDAC5), which was directly bound to and colocalized with TFEB, inhibited TFEB-induced MuRF1/Trim63 expression. The inhibition of TFEB by HDAC5 was reversed by PKD1, which was associated with HDAC5 and mediated its nuclear export. Mice lacking PKD1 in skeletal myocytes were resistant to Ang II–induced muscle wasting. In our study we proposed that elevated Ang II serum concentrations, as occur in patients with congestive heart failure, could activate the PKD1/HDAC5/TFEB/MuRF1 pathway to induce skeletal muscle wasting. Now we are working on further molecular mechanisms involved in this signalling pathway.
The Ang II/PKD1/HDAC5 signaling pathway regulates TFEB-induced MuRF1/Trim63 expression. The PKD1/HDAC5/TFEB/MuRF1 axis mediates Ang II induced skeletal muscle atrophy. Nuclear TFEB specifically binds to conserved E-box motifs in the MuRF1/Trim63 promoter localized close to its transcription start site. PKD1 together with HDAC5 controls TFEB activity at the MuRF1/Trim63 promoter. Inhibition of this signaling pathway could be important to combat Ang II associated muscle wasting disorders such as cardiac cachexia.
- Regulation of function and activity of Muscle RING-finger (MuRF) proteins
-
The Muscle RING-finger (MuRF) Protein Family of E3 Ligases
-
Striated muscle structure and function is maintained by precise control of protein synthesis and degradation; abnormalities in these processes can give rise to myopathies. The UPS degrades misfolded proteins. UPS substrate specificity is mediated by E3 ligases, such as RING-finger proteins. Muscle RING-finger (MuRF) proteins 1, 2, and 3 comprise a subfamily of the RING-finger E3 ubiquitin ligases that are specifically expressed in the heart and skeletal muscle.
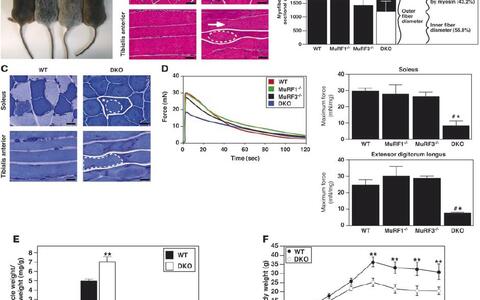
We generated MuRF3 knockout mice and demonstrated that MuRF3 is involved in maintaining cardiac structure and function, and the integrity of the ventricular wall following acute myocardial infarction. Additionally, we found that MuRF1–/–/MuRF3–/– double mutant (DKO) mice display a distinct cardiac and skeletal muscle protein aggregate myopathy. Cardiac and skeletal muscles of DKO mice displayed a striking subsarcolemmal accumulation of myosin (MyHC) accompanied by cardiac hypertrophy, decreased cardiac function and reduced maximal force development of the skeletal muscle (Figure 1). MuRF1 and MuRF3 interact specifically with ?/slow MyHC and MyHCIIa and utilize UbcH5a, -b, and -c as E2 ubiquitin–conjugating enzymes to catalyze the ubiquitinylation and degradation of these proteins. In addition, we generated and phenotypically characterized MuRF2–/–/MuRF3–/– DKO mice. MuRF2–/–/MuRF3–/– DKO mice also showed a protein aggregate myopathy in skeletal muscle. Maximal force development was reduced in DKO skeletal muscle. In addition, a fibre type shift towards slow/type I fibres occurred in MuRF2–/–/MuRF3–/– DKO soleus and extensor digitorum longus. MuRF2–/–/MuRF3–/– DKO hearts showed decreased systolic and diastolic function. Further analyses revealed an increased expression of the MyHC isoform beta/slow and disturbed calcium handling as potential causes for the phenotype in MuRF2–/–/MuRF3–/– DKO hearts. Our data show that all members of the MuRF family have partially redundant functions which are important for maintenance of skeletal muscle and cardiac structure and function in vivo. We are now working on the regulation of function and activity of MuRF proteins.
Double knock-out mice show a skeletal muscle myopathy. (A) Mice at 12 weeks of age. (B) H&E stain and (C) myosin ATPase activity of cross-sections from soleus and longitudinal sections from tibialis anterior muscles from 12-week-old WT and DKO mice. Subsarcolemmal eosinophilic material accumulated along the entire length of the myofiber around a central myofiber core of DKO muscle. Heterogeneity of fiber size, atrophic myofibers (arrowhead), and centrally localized nuclei (long arrow) were found in DKO muscle. Solid lines indicate outer myofiber membrane. Dotted lines define the central core, which is surrounded by accumulated material (asterisk). Short arrow indicates basophilic boundary surrounding inner fiber. Quantitation of myofiber cross-sectional area and determination of occupied surface area of myofibers from soleus muscle (n = 6 each). (D) Measurement of force development and fatigability. Representative recordings of force from soleus muscles. Quantitation of maximum force development from soleus and extensor digitorum longus (n = 6 each). mN, millinewtons. (E) Quantitation of skeletal muscle mass (n = 12 each) of 12-week-old mice. (F) Measurement of body weight with increasing age. Scale bars: 20 μm. #P < 0.01 versus WT and MuRF1–/–; *P < 0.01 versus MuRF3–/–; **P < 0.01 versus WT.
- Inflammation-dependent skeletal muscle failure
-
Inflammation-induced skeletal muscle failure in critically ill patients
-
Clinical data suggested that sepsis and systemic inflammation are major risk factors for ICUAW and CIM and increased serum levels of inflammatory cytokines might induce muscle atrophy in critically ill patients. We reported that the inflammatory cytokine interleukin-6 (IL6) is continuously increased in muscle during inflammation and associated with muscle failure. Increased IL6 expression in muscles of critically ill patients was indicative for inflammation directly occurring in muscle.
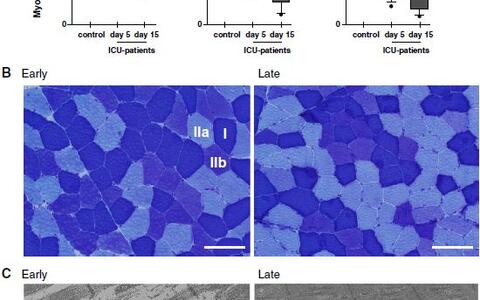
We reported that inflammation causes acute-phase response (APR) directly in skeletal muscle of critically ill patients. The APR protein serum amyloid A (SAA) 1 was increased in the skeletal muscle of CIM patients were it accumulated in the interstitium, around myofibers. Increased SAA1 expression was inversely correlated with excitability of muscle membranes indicating that SAA1 plays a role in muscle function. We showed that differentiated human and murine myocytes synthesize SAA1, and that SAA1 synthesis was increased by inflammatory cytokines (i.e. IL6, TNF?). Importantly, both IL6 and SAA1 induce atrophy by increasing protein degradation in muscle of patients and rodents. Together, our data indicated that inflammation induces APR directly in muscle possibly contributing to ICUAW. However, the mechanisms how inflammatory cytokines and APR proteins mediate muscle atrophy, and the receptors and signaling pathways involved in this process are unknown.
Fast- and slow-twitch Type-IIb fibers atrophied during critical illness. (A) Myocyte crosssectional area (MCSA, lm2) from controls (white) and ICU patients’ first (gray) and second (black) biopsy specimens in type-I (left), type-IIa (middle), and type-IIb (right) fibers. **P\0.01; *P\0.05; n.s. not significant. (B) ATPase stained fiber-type analyses from early (left) and late (right) time points (type-I, -IIa, and -IIb fibers are indicated; scale bar 100 lm). (C) Representative electron micrographs from the early (left) and late (right) time points revealed early destruction of myofiber ultrastructure (scale bar 2 lm). Myosin loss and mitochondrial ballooning occurred early during critical illness (left). At the later time point myosin became squeezed and distorted, Z-lines were deformed and H-zone shapes were blurred (right). Myosin loss (thick black arrow), Z-lines (white arrow), H-zone (small black arrow), mitochondria (white star).
Cooperations, Funding and Job Offers
Cooperation partners
Cooperations at the Max Delbrück Center for Molecular Medicine (MDC), Belin, Germany
•Prof. Dr. Thomas Sommer - Intracellular Proteolysis
•Prof. Dr. Michael Bader - Molecular Biology of Peptide Hormones
•Prof. Dr. Carmen Birchmeier - Developmental Biology / Signal Transduction
Cooperations at the Experimental and Clinical Research Center (ECRC), Berlin, Germany
•Dr. med. Michael Boschmann - Clinical Reseach Unit
Cooperation with our other partners
•PD Dr. Steffen Weber-Carstens, Klinik für Anästhesiologie mit Schwerpunkt operative Intensivmedizin der Charité - Universitätsmedizin Berlin, Berlin, Germany
•Prof. Dr. Hortense Slevogt, Universitätsklinikum Jena, Zentrum für Innovationskompetenz Septomics; Jena, Germany
•Prof. Dr. Kai Kappert, Center for Cardiovascular Research (CCR), Charité - Universitätsmedizin Berlin, Berlin, Germany
•Prof. Dr. Gunnar Dittmar, Luxembourg Institute of Health, Luxembourg
•Prof. Dr. Oliver Müller, Klinik für Kardiologie, Angiologie und Pneumologie, Innere Medizin III, Heidelberg, Germany
•Prof. Dr. Theresia Kraft, Institut für Molekular- und Zellphysiologie, Physiologie, Medizinische Hochschule Hannover, Germany
•PD Dr. Renate Scheibe, Institute for Physiological Chemistry, Gene regulation in cardiomyocytes and in skeletal muscle, Medizinische Hochschule Hannover, Germany
•Prof. Dr. Siegfried Labeit, Department of Integrative Pathophysiology, Universitätsmedizin Mannheim, University of Heidelberg
Funding
- Berlin Institute of Health (BIH)
- Charité-Universitätsmedizin Berlin
- Deutsche Forschungsgemeinschaft (DFG)
- Deutsches Zentrum für Luft und Raumfahrt e. V. (DLR) and European Space Agency (ESA)
- Max Delbrück-Centrum für Molekulare Medizin (MDC)
Job offers
People with brilliant ideas and commitment are always welcome to apply.
We have open positions for master's students and medical doctoral candidates.
Please contact Jens Fielitz for more information.