Only apparently healthy: How titin gene mutations affect heart function
Titin is the largest protein in the human body and is found in muscle tissue. It functions like a spring when the heart muscles contract and relax. If the length of this molecular spring changes, the mechanical properties of the muscle also change – it becomes more rigid, for example, or the heart expands too much and can no longer pump blood efficiently.
As a result of certain mutations in the titin gene, 50 percent of all titin (TTN) proteins in the heart are not produced completely and can thus not be used by the heart. These mutations, called TTNtv (titin truncating variants), are present in 25 percent of all patients with hereditary dilated cardiomyopathy (DCM). DCM is a form of cardiac insufficiency that can lead to heart failure and sudden death.
Two targeted mutations in the titin gene
But many healthy people are also carriers of these mutations, which are very common among the general population. It is thus unclear whether TTNtv necessarily have an adverse effect on heart function. Why are most people with TTNtv healthy – or does the mutation perhaps affect their hearts after all? This is the question addressed in the study.
The MDC team delivered a key component in answering this question. They investigated two rat models on the molecular level: “Genetically, the rats were completely identical apart from a single, targeted mutation in the titin gene that was designed to be in different parts of the gene in the two models,” explains Sebastiaan van Heesch. He is one of several researchers in Norbert Hübner’s MDC working group who were involved in the project.
The heart has to adapt its energy metabolism
“All the rats in our study seemed to be healthy but they showed molecular characteristics that we also found in the hearts of people with DCM,” says the researcher. One of these characteristics is the production of longer titin isoforms as well as the continuous production and subsequent disposal of mutated titin RNA and mutated titin proteins. “These processes cost the heart a considerable amount of energy. We were able to show on the molecular level that the heart has to adapt its energy metabolism to continue to function adequately,” says van Heesch. The changes in the energy balance were later confirmed by the team in Singapore, which also observed that the apparently healthy but mutated rat hearts were unable to contract correctly when they were exposed to targeted stress.
Eleonora Adami, who also worked on the project, adds: “Overall, the heart seems to be in a permanent state of compensation in order to correct the mistakes triggered by the titin mutations. When the heart is exposed to additional stress, heart failure might be triggered.”
To see exactly what is happening here with the titin molecules, the MDC researchers analyzed the processes at the translational level, in other words, the production of protein by the ribosomes. Eleonora Adami has optimized a special technique that makes it possible to measure ribosomal activity directly in the heart tissue. Only the sections of RNA where the ribosomes are located are sequenced. “This technique allows us to visualize the actual translation of the mutated gene,” says Adami. She observed how the ribosomes converted the RNA information into titin proteins, some of which were shortened.
The cell needs to break down "useless" titin proteins
“We were able to show for the first time that not all mutated titin RNA molecules are degraded but that some are translated in the shortened variant. These shorter versions of the titin protein are recognized as mistakes by the cell and need to be broken down. This has an effect on cardiac metabolism; a lot of the cell’s energy needs to be repurposed,” says Adami. “This tells the heart that it has to do something to keep healthy and expend additional energy – which is a further stressor for the heart,” adds van Heesch.
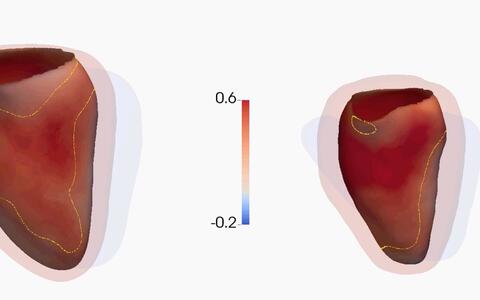
The researchers looked further into the issue and analyzed the data of 2,495 DCM patients. To identify the impact of TTNtv on the heart, the team also sequenced the genes of a further 1,409 healthy individuals and examined their hearts using cardiac magnetic resonance imaging (MRI). On a physiological level, the changes triggered by the titin mutations have the overall effect of weakening the heart muscle; the MRI analyses confirmed this, showing that the apparently healthy test subjects with titin mutation had slightly enlarged, although still correctly functioning, hearts.
Potential use for heart screenings
With this study, the MDC researchers have improved the general understanding of the potential of such titin mutations to cause disease. Their approach focused on the various molecular levels of gene expression, the process in which genetic information is translated and made available for the cells and for the body. “Millions of people around the world have mutations in the titin gene and seem to be healthy, but in combination with the right stress effectors their hearts might fail one day. Our findings can be used in the future for screenings to identify people with an increased risk of developing heart problems,” says Eleonora Adami.
It is still unclear at present which factors can trigger heart disease in people with the titin mutation. These could be additional genetic factors or environmental factors such as alcohol abuse or extreme sports - all of which are interesting questions requiring further research.
1National Heart Centre Singapore, Singapore. 2Duke–National University of Singapore, Singapore. 3Cardiovascular and Metabolic Disorders Program, MRC Clinical Sciences Centre, Faculty of Medicine, Imperial College London, Hammersmith Hospital Campus, London, UK. 4Cardiovascular and Metabolic Sciences, Max Delbrück Center for Molecular Medicine in the Helmholtz Association (MDC), Berlin, Germany. 5National Heart and Lung Institute and NIHR Royal Brompton Cardiovascular BRU, Imperial College London, London, UK. 6Department of Surgery, National University of Singapore, Singapore. 7Departments of Cardiology and Vascular Surgery, University Medical Center, Utrecht, the Netherlands. 8Department of Computing, Imperial College London, London, UK. 9Institute of Gender in Medicine, Charité Universitätsmedizin Berlin, Berlin, Germany. 10DZHK (German Centre for Cardiovascular Research), partner site Berlin, Berlin, Germany. 11Department of Genetics, Harvard Medical School, Boston, Massachusetts, USA. 12Division of Cardiovascular Medicine, Brigham and Women’s Hospital, Boston, Massachusetts, USA. 13Howard Hughes Medical Institute, Chevy Chase, Maryland, USA. 14Department of Cardiovascular Physiology, Ruhr University Bochum, Bochum, Germany. 15DZHK (German Centre for Cardiovascular Research), partner site Goettingen, Goettingen, Germany. 16Charité Universitätsmedizin, Berlin, Germany. 17These authors contributed equally to this work. 18These authors jointly supervised this work