Gene mutation in the chloride channel triggers rare high blood pressure syndrome
The steroid hormone aldosterone, in concert with other mechanisms, controls our blood pressure. It is secreted by the adrenal glands and regulates the water and salt balance in the body. Adrenal glands in patients affected by hyperaldosteronism produce excessive amounts of aldosterone, which leads to excessive sodium retention which in turn increases the excretion of potassium. In the end, this leads to abnormally high blood pressure, ‘arterial hypertension’. The combination of high aldosterone concentration and high blood pressure often results additionally in kidney damage.
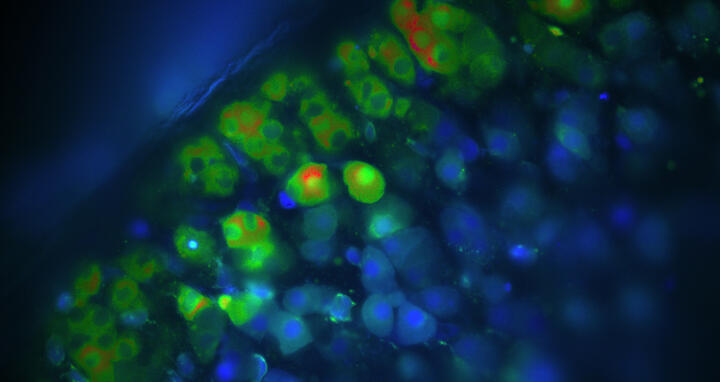
Kalziummessungen von Aldosteron-produzierenden Zellen der Nebenniere. Sind die Zellen blau, ist die Kalziumkonzentration in diesen Zellen niedrig. Grüne und rote Zellen haben eine erhöhte Kalziumkonzentration, sodass sie Aldosteron produzieren.
The pathological mechanisms of the disease, also known as Conn syndrome, remained incompletely understood. In 2018, Paris-based scientists around Prof. Maria-Christina Zennaro teamed up with colleagues from the Leibniz-Forschungsinstitut für Molekulare Pharmakologie (FMP) and the Max Delbrück Center (MDC) as well as other scientists in Germany and the USA around Prof. Ute Scholl from the Berlin Institute of Health (BIH) and Charité, and found mutations in the ClC-2 chloride channel in patients affected by this syndrome. To date, a total of six different mutations have been described (published in Nature Genetics 2018). However, the pathway leading from the mutations to aldosterone overproduction had remained unclear - until researchers from FMP and MDC generated and analyzed a specific mouse model.
Proven causality between mutation and disease
The team, led by Prof. Thomas Jentsch - a pioneer who discovered the first chloride channel family, including ClC-2, almost three decades ago, initially investigated all known aldosteronism-causing ClC-2 mutations in vitro. They found that all these mutations drastically increased the flow of chloride through the channel.
To examine the hypothesis that increased chloride flow through ClC-2 causes hyperaldosteronism, the researchers then developed a mouse model in which ClC-2 was activated by an 'artificial' mutation that had not been reported for patients. The genetically modified mice exhibited enormously increased chloride currents in aldosterone-secreting cells, which indirectly led to a large increase in aldosterone concentration in the blood of those rodents. Just like in patients, this resulted in abnormally elevated blood pressure and secondarily reduced activity of renin, a hormone that normally boosts aldosterone production. In addition to proving that an increase in Cl- currents in adrenal gland cells leads to hyperaldosteronism, the researchers investigated the pathological pathway in great detail.
Chloride channel continuously open
"We have seen how the channel is constantly open due to these mutations, which greatly changes the electrical voltage across the membrane of the hormone-producing cell. This leads to an influx of calcium, which, in turn, causes overproduction of aldosterone," explains Dr. Corinna Göppner, who, together with Dr. Ian Orozco, is the first author of the article which was just published in Nature Communications.
"We have been able to show step by step what exactly happens in the organism due to the mutated chloride channel in our model for the first time," said the biologist. "In this respect, our work has excellently complemented and expanded the human genetic findings."
The best model to explore hyperaldosteronism
The mouse model developed in Berlin Buch is the first in-vivo model that faithfully models all symptoms of the disease. Therefore, it is the perfect model to investigate the pathological mechanisms of hyperaldosteronism and to identify secondary effects such as long-term damage. The researchers were already able to detect slight kidney damage, but they hope more effects will be discovered: "At the moment, we assume that a constitutively open chloride channel could also impact other organs," says group leader Thomas Jentsch. Unfortunately, this is still a grey area in medicine, although the long-term consequences are highly relevant for patients. "Our mouse model can definitely help to clarify the situation. This reaffirms once again, how relevant basic research can be for clinical applications." (fmp)
Further information
Publication
Corinna Göppner, Ian J. Orozco et al. (2019): “Pathogenesis of hypertension in a mouse model for human CLCN2 related hyperaldosteronism”. Nature Communications, DOI: 10.1038/s41467-019-12113-9.
Contacts
Prof. Thomas J. Jentsch
Head of Lab "Physiology and Pathology of Ion Transport"
Leibniz-Forschungsinstitut für Molekulare Pharmakologie (FMP) and Max Delbrück Center for Molecular Medicine in the Helmholtz Association (MDC)
+49-30-94793-104
Jentsch@mdc-berlin.de
Silke Osswald
Public Information Officer
Leibniz-Forschungsinstitut für Molekulare Pharmakologie (FMP)
+49-30-94793-104
osswald@fmp-berlin.de
Jana Schlütter
Editor, Communications Department
Max-Delbrück-Centrum für Molekulare Medizin in der Helmholtz-Gemeinschaft (MDC)
+49(0)30-9406-2121
jana.schluetter@mdc-berlin.de
- The Max Delbrück Center for Molecular Medicine (MDC)
-
The Max Delbrück Center for Molecular Medicine in the Helmholtz Association (MDC) is one of the world’s leading biomedical research institutions. Max Delbrück, a Berlin native, was a Nobel laureate and one of the founders of molecular biology. At the MDC’s locations in Berlin-Buch and Mitte, researchers from some 60 countries analyze the human system – investigating the biological foundations of life from its most elementary building blocks to systems-wide mechanisms. By understanding what regulates or disrupts the dynamic equilibrium in a cell, an organ, or the entire body, we can prevent diseases, diagnose them earlier, and stop their progression with tailored therapies. Patients should benefit as soon as possible from basic research discoveries. The MDC therefore supports spin-off creation and participates in collaborative networks. It works in close partnership with Charité – Universitätsmedizin Berlin in the jointly run Experimental and Clinical Research Center (ECRC), the Berlin Institute of Health (BIH) at Charité, and the German Center for Cardiovascular Research (DZHK). Founded in 1992, the MDC today employs 1,600 people and is funded 90 percent by the German federal government and 10 percent by the State of Berlin.
Das Leibniz-Forschungsinstitut für Molekulare Pharmakologie (FMP)
Das Leibniz-Forschungsinstitut für Molekulare Pharmakologie (FMP) gehört zum Forschungsverbund Berlin e.V. (FVB), einem Zusammenschluss von acht natur-, lebens- und umweltwissenschaftlichen Instituten in Berlin. In ihnen arbeiten mehr als 1.900 Mitarbeiter. Die vielfach ausgezeichneten Einrichtungen sind Mitglieder der Leibniz-Gemeinschaft. Entstanden ist der Forschungsverbund 1992 in einer einzigartigen historischen Situation aus der ehemaligen Akademie der Wissenschaften der DDR.