Why APOE4 raises Alzheimer’s risk
The gene variant APOE4 has long been known as the strongest genetic risk factor for late-onset Alzheimer’s disease, raising the risk about twelve-fold as compared to non-carriers. Yet its close relative, APOE3 – the most common variant in humans – does not appear to increase susceptibility to the disease. The reason for this stark difference has been unclear.
Now, a study published in “Nature Metabolism” may explain the mechanism: Neurons exposed to APOE3 protein can use long-chain fatty acids as an alternative source of energy when glucose is scarce. But this vital metabolic pathway is blocked in the APOE4 brain.
“The ability to use glucose diminishes in the aging brain, forcing nerve cells to use alternative sources for energy production,” explains Professor Thomas Willnow, Group Leader of the Molecular Cardiovascular Research lab at the Max Delbrück Center and senior author of the paper. Willnow also holds a professorship in the Department of Biomedicine at Aarhus University. “APOE4 appears to block nerve cells from utilizing lipids as an alternative energy source when their supply of glucose decreases.”
Experiments in mice and human neurons
The brain consumes around a fifth of the body’s glucose supply. Yet as we age, its ability to metabolize glucose drops. This decline is a hallmark of both normal aging and Alzheimer’s disease, and starts decades before symptoms of dementia become apparent.
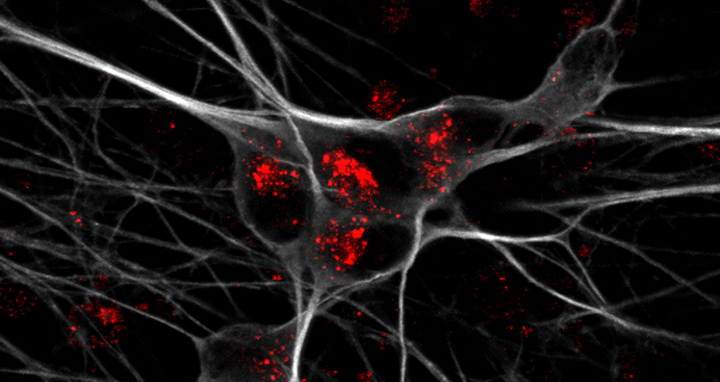
Detection of apoE receptor sortilin (red) in iPSC-derived human neurons (grey).
ApoE, the protein encoded by the APOE gene, belongs to a family of fat-binding proteins, called apolipoproteins. In the central nervous system, ApoE is mainly released by brain cells called astrocytes. It helps to deliver lipids to nerve cells.
To understand why the APOE4 variant so dramatically raises the risk of Alzheimer’s disease compared to APOE3, co-first authors Dr. Anna Greda, assistant professor at the Willnow lab at Aarhus University, and Dr. Jemila Gomes, who did her PhD in Aarhus and is now a postdoc in the Willnow lab in Berlin, collaborated with the Technology Platforms for Pluripotent Stem Cells and Electron Microscopy at the Max Delbrück Center. They used genetically engineered mice that carry human APOE3 or APOE4 genes. They found that APOE3 interacts with a receptor called sortilin to deliver fatty acids into neurons. By contrast, APOE4 disrupts sortilin’s function, preventing uptake of lipids by neurons.
To confirm the relevance of their findings for human brain health, the scientists then turned to human stem cell-derived neurons and astrocytes carrying different APOE variants. Again, they observed that APOE3 allowed neurons to metabolize long-chain fatty acids, while the presence of APOE4 shut down this ability.
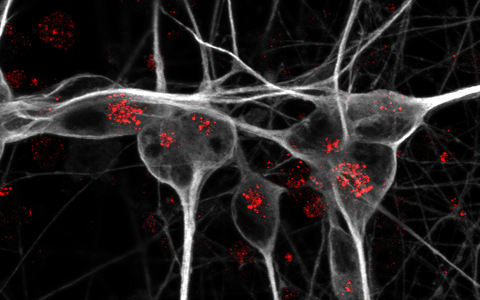
Detection of mitochondria (red) in cell body and neurites of iPSC-derived human neurons (green).
“By using transgenic mouse models and stem-cell-derived human brain cell models, we discovered that the pathway enabling nerve cells to burn lipids for energy production doesn’t work with APOE4, because this APOE variant blocks the receptor on nerve cells required for lipid uptake,” explains Greda.
New treatments for Alzheimer’s
“Our research suggests that the brain is highly dependent on being able to switch from glucose to lipids as we age. It seems that individuals, who are carriers of the APOE4 gene, may be compromised to do so, increasing their risk of nerve cell starvation and death during aging,” Gomes adds. Still, “this work opens new avenues for interventions that could improve lipid-based energy use in APOE4 carriers.”
There are already marketed drugs that specifically target the body’s ability to utilize lipids, Willnow says. Such drugs can now be studied for their potential to treat people with APOE4. As a proof, the researchers showed that treating neurons with the drug bezafibrate restored fatty acid metabolism in APOE4-expressing cells. Of course, such drugs need to be tested in clinical trials, Willnow adds, “but I am hopeful that our research suggests new treatment options for this devasting disease.”
Text: Gunjan Sinha / Vibe Bregendahl Noordeloos, Aarhus University
Further information
Literature
Anna Greda, Jemila Gomes, et al. (2025): „Interaction of sortilin with apolipoprotein E3 enables neurons to use long-chain fatty acids as alternative metabolic fuel“. Nature Metabolism, DOI:10.1038/s42255-025-01389-5
Photo for download
Detection of apoE receptor sortilin (red) in iPSC-derived human neurons (grey). Photo: Anna K Greda, Universität Aarhus
Detection of mitochondria (red) in cell body and neurites of iPSC-derived human neurons (green). Photo: Anna K Greda, Universität Aarhus
Contact
Professor Thomas Willnow
Group Leader, Molecular Cardiovascular Research lab
Max Delbrück Center
willnow@mdc-berlin.de
Jana Schlütter
Editor and Deputy Head
Max Delbrück Center
+49 30 9406-2121
jana.schluetter@mdc-berlin.de or presse@mdc-berlin.de
- Max Delbrück Center
-
The Max Delbrück Center for Molecular Medicine in the Helmholtz Association lays the foundation for the medicine of tomorrow through our discoveries of today. At locations in Berlin-Buch, Berlin-Mitte, Heidelberg, and Mannheim, interdisciplinary teams investigate the complexity of disease at the systems level – from molecules and cells to organs and entire organisms. Together with academic, clinical, and industry partners, and as part of global networks, we turn biological insights into innovations for early detection, personalized therapies, and disease prevention. Founded in 1992, the Max Delbrück Center is home to a vibrant, international research community of around 1,800 people from over 70 countries. We are 90 percent funded by the German federal government and 10 percent by the state of Berlin.