Cell cycle surprises
The cell cycle, the process through which cells multiply, has evolved to be as simple as possible by minimizing potential for errors. An interdisciplinary team of researchers from the Max Delbrück Center for Molecular Medicine in the Helmholtz Association (MDC) report this in the journal Molecular Systems Biology.
Cells multiply by going through a four-step cycle: expanding in size, copying their genetic information, preparing to divide, and then finally dividing into two cells. Textbooks typically display this by a simple circle, but for no particular scientific reason. For the first time, a study shows that this depiction is justified, as cells do display a circular pattern of gene expression while going through the cell cycle. It also shows that this is not a coincidence; this pattern significantly reduces how many mistakes can occur in the process – an evolutionary achievement.
“The cell cycle is one of the most studied processes in biology,” says Professor Nikolaus Rajewsky, Scientific Director of MDC’s Berlin Institute for Medical Systems Biology (BIMSB), who spearheaded the project. “The power of ultra-deep, single-cell sequencing combined with computational approaches has allowed us to look at the cell cycle in greater detail and make new unexpected discoveries.”
Big, big data
The findings come thanks to Rajewsky and molecular biologists in his Systems Biology of Gene Regulatory Elements Lab at BIMSB in the city center, teaming up with a physicist and a mathematician in the Mathematical Cell Physiology Lab at Buch campus.
Rajewsky and his colleagues sequenced messenger RNA (mRNA) for thousands of individual cells throughout the entire cell cycle. Levels of mRNA indicate which genes are active and what the cell is doing. Paper co-author Sara Formichetti led these “ultra-deep sequencing” efforts and gained more detailed information about gene activity in each cell than previously possible. Usually, there is a trade off in sequencing studies – either scientists can sequence a lot of cells, or they can get a lot of information per cell. Formichetti optimized to get the best of both worlds, building a detailed dataset that covered the whole cycle.
Formichetti and Rajewsky began to computationally sort the cells along the cell cycle time, but Formichetti’s guest visit in the Rajewsky lab ended and so Rajewsky reached out to Professor Martin Falcke, who heads the Mathematical Cell Physiology Lab, and first paper author Daniel Schwabe, a Ph.D. candidate in his lab.
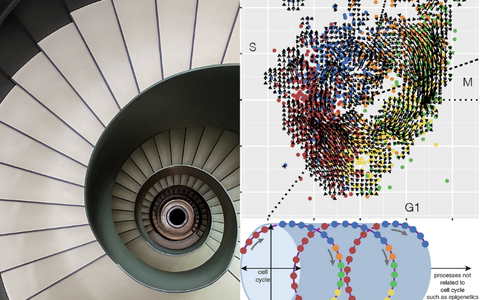
Cells are capable of performing multiple processes at the same time. If we isolate the process of the cell cycle, then the inner engine of the cell performs a motion which we can compare to walking in a circle, round and round. However, in reality, other biological processes are causing additional movement, that are uncoupled from the cell cycle, in a third dimension. This is as if we continue walking in a circular motion but we are now walking on a spiral staircase. Once we complete one round, we find ourselves one level above or below from where we started.
Geometry for biology
The team wanted to see if the data cloud would form a geometric shape reflecting the cell cycle process. They expected something like a messy knot of string, which would correspond to many genes being turned on and off multiple times throughout the cycle.
At first, they didn’t see any pattern or shape. So, they began to rotate the data cloud to view it from different angles. Imagine holding a mug or cup, turning it around in your hands to view it from all sides, lifting it up to see the bottom and then peering down at it from the top. The researchers did essentially the same thing and discovered that from a particular angle, the data forms a hollow cylinder, and, like when looking at the mug straight down, a circle that corresponds to the cell cycle – the simplest shape possible.
“There was no evidence that would indicate the cell cycle would be this simple,” Schwabe says. “What this tells us is that each gene involved in the cell cycle is turned on and off only once throughout the cycle.”
Since turning genes on and off only once helps reduce potential for errors, it suggests the cell cycle has evolved to be as simple and efficient as possible. Furthermore, in the immortalized cell lines they studied, the researchers found that the cell cycle is basically independent of other biological processes active in the cell, which makes it even more robust against errors.
“The level of optimization and isolation we observe is remarkable,” Rajewsky says. “On the one hand, we completely capture the biology of the cell cycle in a two-dimensional circular motion while at the same time processes such as epigenetics and changes in the environment push the cells along a perpendicular axis. Taken together, these two influences cause a spiraling motion along a hollow cylinder.”
“On the fly”
You would expect the cell to stop [...], but that is not the case. This occurs on the fly, while the engine keeps running.
The researchers made another unexpected discovery. The cell cycle is known to have check points, at which cells make sure all necessary steps are complete before moving onto the next phase of the cycle. They expected that gene activation, during which the gene is transcribed into mRNA, slows down before check points, providing well-defined moments for determining whether or not to proceed. But the data here show a continuous, relatively even rate of gene expression activity, with no defined breaks.
“I was surprised,” Falcke says. “You would expect the cell to stop when it checks that everything on its to do list has been completed, but that is not the case. This occurs on the fly, while the engine keeps running.”
The revelations may not stop there. The team is able to use their approach to precisely remove cell cycle effects from data sets, leaving only the irregularities, something of great interest to researchers in similar fields. For example, it could help clarify what goes wrong in the cycle that enables unchecked cell division and tumor growth. Rajewsky and Falcke plan to continue using it to explore the origin of cell variability, which is what drives or enables clonal cells to have very different gene expression levels, as well as other questions.
Text: Laura Petersen
Further information
Literature
Schwabe, Daniel et al. (2020): „The transcriptome dynamics of single cells during the cell cycle“. In: Molecular Systems Biology; DOI: 10.15252/msb.20209946
Downloads
This spiral staircase at BIMSB represents that gene expression of the cell cycle moves along a spiral on a hollow cylinder when it is combined with additional biological processes. Foto: Valentin Popescu, MDC