Challenging assumptions in immunology and DNA repair
The fields of immunology and DNA repair need to rethink how a key protein supports the body’s immune system, according to new research published in the journal Cell Reports.
The protein 53BP1 is critical for producing different antibody types during immune responses. For many years, scientists thought the protein primarily protects the ends of broken DNA strands from further damage during the normal process of producing antibodies. MDC researchers have found that this is not the protein’s essential role.
It’s not every day a research team uncovers something that challenges the way a field thinks. Indeed, it was hard to embrace their own study results when they were so unexpected. “It took a long time for me to accept this,” said Dr. Michela Di Virgilio, head of the MDC Laboratory for DNA Repair and Maintenance of Genome Stability and junior professor at the Charité. “For us it is big deal. We are changing the direction we’re taking in the lab.”
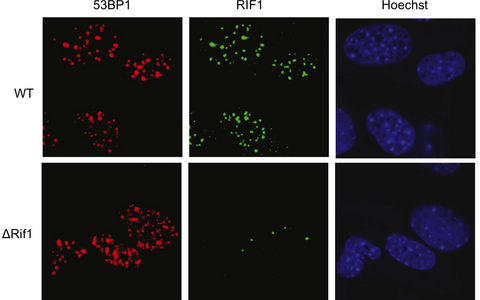
Researchers used fluorescently tagged antibodies to track 53BP1 (red) and another key associated protein, RIF1 (green), to see if the proteins were recruited to areas of DNA damage inside the cell nucleus (blue).
A closer look
The group was investigating the role of 53BP1 in a vital process for robust immune responses called immunoglobulin class switch recombination (CSR). During CSR, the antibody-producing cells in our body, called B lymphocytes, break their own DNA, cut out part of the sequence, and then rejoin to produce antibodies better suited to fight pathogens. CSR results in antibodies that attack pathogens in different ways, and are therefore better equipped to dispose of them. Patients without this CSR ability have severely compromised immune systems and have a very hard time fighting infections.
Broken DNA is usually very dangerous, opening the door to mutations that are more likely to lead to cancer or cell death. The fact that immune cells break their DNA on purpose – not just one, but both strands – fascinates Di Virgilio. “This damage is the worse type of DNA damage we can have,” she said. “But in this case, the cell is breaking its DNA on purpose for the benefit of immunity.”
A schematic representation of the 53BP1 protein, which is known to play an important role during DNA damage response and immunity.
One door closes, another opens
Protein 53BP1 does indeed protect broken DNA strands from being damaged further before rejoining is complete. To see whether this function is essential for CSR, the team used genetic and biochemical tools to prevent 53BP1 from protecting the DNA ends, without affecting its other known functions. They were surprised to see that CSR was only marginally affected. When they knocked out 53BP1 completely, CSR was nearly stopped, as expected. This indicates that the protein is critical, but not in the way everyone thought.
“This study opens up a new door for scientists in our field to rethink the pathway towards antibody diversification and how 53BP1 could achieve this feat,” said Devakumar Sundaravinayagam, MDC researcher and first author of the paper.
Still a mystery
So what is the protein’s key function in the process? That is still to be determined. One possibility raised by others in the field is the protein somehow helps maintain the functional structure or architecture of the immunoglobulin gene. Di Virgilio and her team believe this function might be responsible for the crucial role 53BP1 plays in CSR. Studies are already under way to find out if this is the correct answer.
Figuring out the nitty gritty details of the DNA damage and repair process is hardcore, basic molecular biology. But it can have immense benefits for patients down the road. “I love it. It’s really cool,” Di Virgilio said. “The translational potential is great in the long term, but we first need to understand the underlying mechanisms before we can move on.”
Laura Petersen
Further information
Michela Di Virgilio arwarded junior professorship
The immune system as a sculptor
Literature
Devakumar Sundaravinayagam et al. (2019): „53BP1 supports Immunoglobulin Class Switch Recombination independently of its DNA Double-Strand Break End Protection function“. Cell Reports, doi: 10.1016/j.celrep.2019.06.035