Michael Robson awarded grants to study chromatin
Long before humans walked the Earth, research suggests our mammalian ancestors foraged in darkness. With dinosaurs ruling the daytime, early mammals likely evolved to avoid them by being active at night. Over time, as the dinosaurs vanished and the pressure to stay hidden eased, many mammals – including our own lineage – evolved to see better in daylight. Meanwhile other mammals such as mice, retain remnants of our evolutionary legacy – their eyes are still better adapted to see in darkness. For Dr. Michael Robson, this evolutionary divergence has become the key to unlocking how chromatin – the complex of DNA, proteins, and RNA found in the nucleus of eukaryotic cells – controls gene activity.
With two new research grants – a €449,125 award from the German Research Foundation (DFG) and a $1.5 million project grant from the Human Frontier Science Program (HFSP) that will be split equally among four collaborating labs – Robson’s lab is studying the nature of chromatin – and how disruption in its structure may contribute to human diseases such as Macular Degeneration and Retinitis Pigmentosa.
A nod to our evolutionary past
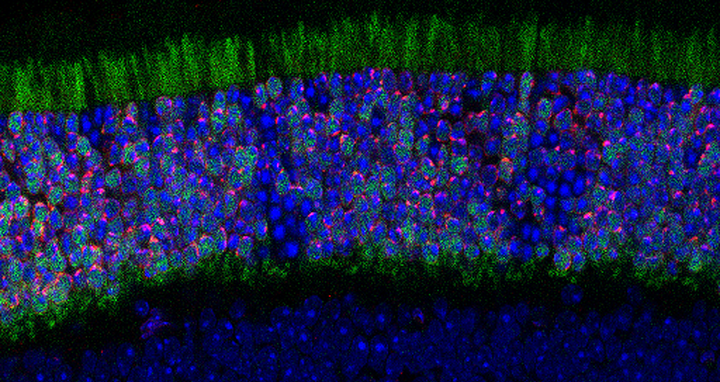
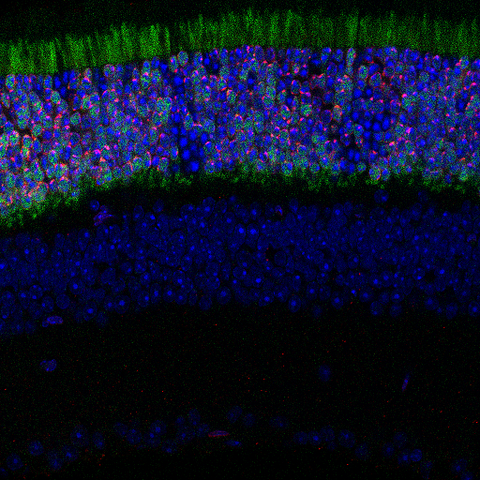
Robson takes advantage of a unique feature of the rod cells in the eyes of mice. In a nod to our evolutionary past, chromatin in these cells is organized to enable the animals to see in the dark – active DNA called euchromatin is pushed to the edge of rod photoreceptor cell nuclei while inactive chromatin, called heterochromatin, is clumped in a dense blob in the center.
A cross-section of retinal tissue from a transgenic mouse genetically engineered to express the chromatin–nuclear lamina tethering protein LBR in rod photoreceptors. In the topmost layer, LBR protein tethers heterochromatin to the nuclear periphery, reverting the inverted nuclear architecture typical of nocturnal rod cells to a more conventional, diurnal-like organization (red). Rods not expressing LBR retain their native inverted chromatin organization (dense blue). Green is used to mark the LBR gene.
This lies in contrast to the rod cells in mammals that are active during the day, including humans, where the organization of euchromatin and heterochromatin is flipped – inactive, tightly packed heterochromatin sits at the edge of the nucleus, while active euchromatin floats in the middle of the cells. The heterochomatin is anchored to the nuclear membrane by specific proteins. Research suggests that some human disorders that affect vision might be caused by dysregulation of these proteins.
But while some proteins have been identified, “we don't know which protein attaches which genomic regions to the periphery,” Robson explains. Because mice lack this interaction in their rod cells, the Robson lab will use genetic engineering techniques to add specific proteins back into mice, and single-cell genomics to understand how each protein regulates the structure of chromatin, and hence gene transcription.
HFSP grant to elucidate how chromatin behaves
With his HFSP is grant, which will be shared among collaborators in Germany, the U.K., the U.S., and Portugal, the Robson group will again take advantage of another unique feature of
mouse eyes: Mice are actually born with rod photoreceptor cells that are structured like their mammalian cousins that see better in daylight. But the cells change even before they are able to open their eyes, within a span of 48 hours, says Robson. This rapid inversion between heterochromatin and euchromatin in rod cells creates a rare window during which the genome is actively rearranging. He and his collaborators aim to investigate a more fundamental question: How does chromatin actually behave? “Does it have properties of a liquid, a gel, or a solid? We can use this rare adaptation when chromatin is moving over time to better understand its physical properties,” he explains. Such physical properties affect how genes are turned on and off.
Using advanced imaging and computational modeling, the team will simulate how different physical states – liquid-like versus solid-like – predict the motion of DNA during this reorganization. “It’s a chance to define the material nature of chromatin,” Robson says, “which ultimately tells us how it works as a machine.”
Text: Gunjan Sinha