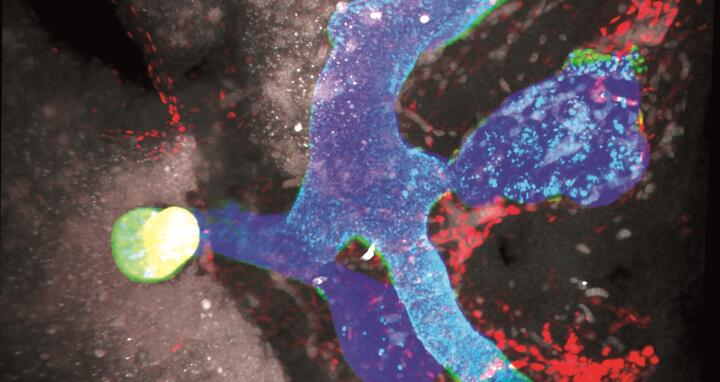
The regenerative power of progenitors
The gastrointestinal tract is like a power plant that converts food into energy for the body. In this biological factory, the liver, gallbladder and pancreas are the assembly line workers that make the whole process possible in the first place and keep it running: They produce the secretions needed to break down food into nutrients in the gut, where it is then fed into the bloodstream.
Francesca Spagnoli has been researching the mechanisms involved in the development of liver and pancreas for many years.
The three organs perform this vital task in close proximity to one another in the upper abdominal cavity. They seem to influence each other already during embryonic development. The pancreas forms from two distinct outgrowths situated opposite each other in the region of the future duodenum along the alimentary canal – what later becomes the gastrointestinal tract. One of these pancreatic outgrowths develops close to the buds of the future liver, gallbladder and bile ducts.
Contrary to previous assumptions, the organs do not form independently of one another. A research group led by Professor Francesca Spagnoli at King’s College London has found that they arise from a common pool of progenitors that retain their plasticity during organogenesis – the formation of organs. Before joining King’s College, Francesca Spagnoli headed the Molecular and Cellular Basis of Embryonic Development Lab at the Max Delbrück Center for Molecular Medicine in the Helmholtz Association (MDC).
Harnessing cell plasticity for regenerative therapies
We suspect that the multipotent progenitor cells maintain their differentiation potential through signals coming from the adjacent intestine.
The researchers have now reported their findings in the journal “Nature”. “We suspect that the multipotent progenitor cells maintain their differentiation potential through signals coming from the adjacent intestine,” says first author Dr. David Willnow. At the time of the study, he was a PhD student at the Berlin Institute of Health (BIH) and a member of the MDC lab of Francesca Spagnoli. He has since followed her to London. For several years Spagnoli has been researching the mechanisms at the origin of the liver and pancreas. She is particularly interested in questions like: How do distinct cell types, such as liver and pancreas, arise from common progenitors and acquire specialized shape to form functional organs? How plastic are these cellular states? Can we harness cell plasticity between liver and pancreas towards novel regenerative therapies for diabetes?
With the study now published in “Nature”, the researchers expand the current understanding of the cellular processes driving early organogenesis. Maintaining multipotent progenitor cells in a developing organ may serve a similar purpose as maintaining a stem cell niche in an adult tissue. A stem cell niche is a region within an organism where stem cells reliably perform the task of replenishing cells. They can transform into progenitor cells that give rise to differentiated cells that engraft into the liver. By being able to renew themselves indefinitely, stem cells along with progenitor cells ensure the physiological balance of an organism: They can replace dead cells and induce tissue regeneration. They also improve resilience during organogenesis – for example, following developmental delay or loss of a lineage-restricted cell population. Failure to maintain such a multipotent progenitor domain might result in human genetic syndromes as well as human malformations in the liver, pancreas and gallbladder.
Illuminating the mechanisms underlying malformations
We developed computer models that quantitatively describe the temporal development of the different cell populations based on the biologically possible scenarios.
To demonstrate that multipotent progenitor cells have the potential to populate the area of tissue from which the liver, gallbladder and pancreas arise, Willnow and the Spagnoli lab combined computational modeling and mouse genetics approaches. The computer models used here were constructed by a team led by Professor Jana Wolf, who heads the Mathematical Modelling of Cellular Processes Lab at the MDC. “We developed computer models that quantitatively describe the temporal development of the different cell populations based on the biologically possible scenarios,” reports Wolf. “Parameterization of these models using experimentally collected data led to the prediction that there must be plasticity between cell populations, which was confirmed in the animal model.”
“In the next step we will study plastic multipotent cell populations in humans, with the aim of exploiting their potential for regenerative medicine,” says Willnow. This might also help to elucidate the mechanisms underlying human genetic syndromes as well as human malformations in the liver, pancreas and gallbladder.
Further information
Dr. Francesca Spagnoli, King’s College London
Literature
David Willnow et al (2021): Quantitative lineage analysis identifies a hepato-pancreato-biliary progenitor niche. Nature, DOI: doi.org/10.1038/s41586-021-03844-1
Download
Light sheet microscopy image of the hepato-pancreato-biliary organ system and gastrointestinal tract of a mouse embryo, immunostained for the transcription factors Pdx1, Sox1 and CK19. Photo: Willnow, Spagnoli, KCL