Sibling Rivalry: how therapies for B cell lymphomas may give rare tumor cells an edge
B cells require surface molecules called B cell receptors (BCRs) to survive. Most tumor cells that arise from B cells seem to need them as well, but rare exceptions may escape therapies. After 15 years, scientists finally understand why.
The paper had been accepted by the journal Nature and was set to appear within days. In 2004 Klaus Rajewsky was on his way to a seminar at Washington University in St. Louis to talk about his group's results, which might represent a major breakthrough in treating cancers called B cell lymphomas. But back in his lab at Harvard, a postdoc named Stefano Casola was about to bring the entire story crashing down. Rajewsky and Casola had realized that the group had forgotten a control experiment. Although the journal hadn't caught the oversight, this meant that a major assumption of the paper had gone untested. That wouldn't do, Rajewsky said – it needed to be checked.
The bad news came when he touched down in St. Louis: the control experiment had partially failed, calling their results into question. This led to the halting of the publication. After making the call, Rajewsky had to completely reorganize the talk he was set to give later that day. The project had suffered a major setback, and the work had to be started from scratch. In Harvard the group went back to the drawing board, and the project continued under Casola’s leadership when he set up his own group at the FIRC Institute of Molecular Oncology in Milan, Italy, in 2006.
Now, 15 years after its initiation, the project has reached maturity in a publication that appears in Nature. The work resolves a basic puzzle concerning B cell lymphomas and offers a cautionary tale for cancer therapies.
BCRs: just how essential are they?
Over a career in research spanning more than 50 years, Rajewsky has been working on the mechanisms that control the immune system, first at the University of Cologne, then at Harvard, and now at the MDC. There are few absolutes in a system that includes dozens of types of cells which interact in highly complex ways to govern our health. But one principle that seemed to be clear was that the survival of B cells depended on a specific molecule on their surfaces.
These B cell receptors (BCRs) represent a form of antibody that is produced by the cell and remains attached to its surface. When a BCR binds to a foreign antigen, it triggers reactions within the cell that play a key role in immune defenses. Some of the cells begin replicating very quickly, becoming factories that churn out vast quantities of the antibody and secrete it into the bloodstream. This instructs other immune cells to recognize and destroy the invading pathogen. Other B cells maintain a "memory" of the infection so that if it ever reappears, the body can mount a swift response.
"Fifteen years ago our hypothesis was that since B cell lymphomas arise from B cells and therefore express B cell receptors, they may also require the receptors for survival," Rajewsky says. "That would represent a weak point you might be able to exploit in a therapy."
Over the years, this idea has formed the basis of many attempts to treat lymphomas by targeting BCRs or the machinery by which they transmit signals into cells. But despite some encouraging results, the ultimate success of such therapies depends on the validity of that initial assumption. And now both its validity and its limitations have finally been determined, at least for one particular type of lymphoma.
Growth despite loss of the receptor
The current study in Nature concerns a particular type of B cell lymphoma which arises through the hyperactivity of an "onco-protein" called Myc. Casola's work shows that if these lymphoma cells lose the BCR, they continue to grow – but they aren't as "fit" as those that do retain their receptors. This result has potential consequences for clinical settings. In a tumor containing both types of cells, those carrying the receptor on the surface will overgrow those without it. If the competition is removed – for example, by a therapy that eliminates cells with BCRs – those that have lost it may take over, to produce tumors that are just as malignant.
To study this problem, the scientists had to build a switch into the genes controlling the cells' ability to produce BCRs. The development of this technology is also a product of pioneering work by Rajewsky and his colleagues, carried out since the late 1980s. It represented a huge leap over other common methods of "knocking out" genes, which eliminated molecules in embryonic cells and thus in the entire organism throughout its lifespan. Such methods made it often impossible to study the roles of genes in adult organisms, because many molecules had essential functions early in life. Knocking them out in embryos often prevented an animal from developing normally in the first place.
Conditional knockouts allowed scientists to eliminate a gene's functions in specific tissues and at any time in the animal's lifespan. In the current study, this permitted the researchers to remove the BCR in established tumor cells, which provided a way to study the impact of the receptor on the cells' biology and functions.
Rewiring tumor metabolism
Casola, with crucial help from MDC scientists Stefan Kempa and Christin Zasada, discovered that the loss of the BCR caused the cells to rewire a fundamental aspect of their metabolism: specifically, the way they derived energy from carbon. This partly explained why the cells weren't as competitive as their counterparts, because it made the cells highly sensitive to situations in which they were deprived of nutrients. The scientists traced this sensitivity to the activity of another molecule called GSK3β. This protein stands at an important intersection between different signaling pathways that control the growth of this type of B-cell lymphoma.
"These tumors arise in cells which are unable to limit their production of the c-Myc oncoprotein, and the result is uncontrolled cell proliferation," Casola says. "That growth is fueled by a substantial reprogramming of the metabolism of malignant cells." But the network of genes controlled by c-Myc is dampened by the activity of the GSK3β protein – it reduces the rate of their growth. Since tumor cells without BCRs produced GSK3β, this explained their lower rate of reproduction. As a result, they were less competitive than cancer cells with the receptors.
The connection between GSK3β and Myc suggested another experiment: What would happen if you lowered the activity of GSK3β in cells without the receptor? The scientists inhibited the activity of the molecule in lymphoma cells. They observed that Myc functions improved, which reversed the dampening of the cells' metabolism. This restored the competitiveness of malignant B cells without BCRs and the cancer thrived.
Casola and his colleagues report that human cells behave like those in the mouse model. In around one third of the human MYC-driven Burkitt lymphoma cases they analyzed, they found a fraction of tumor cells that lacked the BCR. "This discovery warns that treatments which exclusively target the BCR could give rise to recurrences of the lymphoma," Casola says.
These findings provide a rationale to design anti-lymphoma therapeutic protocols where BCR inhibitors are combined with drugs such as rapamycin and MEK kinase, which target the secondary population of tumor cells that lack the BCR. "This makes it essential to find additional weaknesses in cells that lack the receptor,” Casola says.
Varano G, Raffel S, Sormani M, Zanardi F, Lonardi S, Zasada C, Perucho L, Petrocelli V, Haake A, Lee AK, Bugatti M, Paul U, Van Anken E, Pasqualucci L, Rabadan R, Siebert R, Kempa S, Ponzoni M, Facchetti F, Rajewsky K, Casola S. (2017): “The B-cell receptor controls fitness of MYC-driven lymphoma cells via GSK3β inhibition.” Nature. doi:10.1038/nature22353
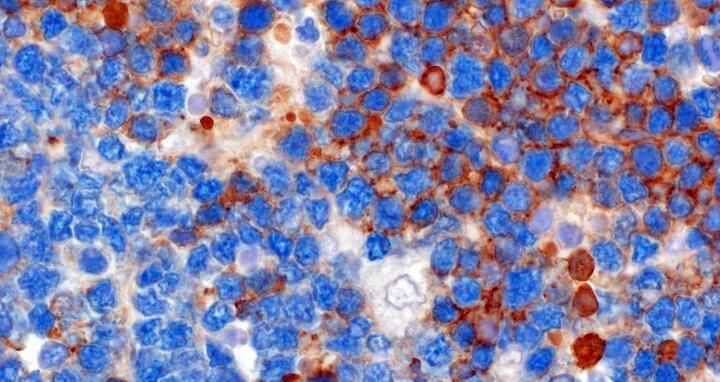
Featured image: Human cells with Burkitt lymphoma may have BCRs (brown) but can also survive without (blue).