T cells require healthy “power plants”
Joint press release by BIH, Charité und Max Delbrück Center
The genome of the mitochondria has large gaps (deletions) in these patients, which results in the cells not having enough energy to perform their various functions.
Patients with Pearson syndrome suffer from anemia because their bone marrow produces too few red blood cells. Immune system defects are also suspected, but these have not yet been studied in detail. The source of these problems are errors in the genome of the cellular power plants, the mitochondria, explains Dr. Leif S. Ludwig, head of the Emmy Noether Independent Junior Research Group “Stem Cell Dynamics and Mitochondrial Genomics” at the Berlin Institute of Health in der Charité (BIH) and Max Delbrück Center. There are often more than 100 mitochondria per cell and each possesses their own genome, which in turn contains genes responsible for energy production. “The genome of the mitochondria has large gaps (deletions) in these patients, which results in the cells not having enough energy to perform their various functions.”
No mutations in the mitochondria of some T cells
Ludwig’s group is part of the joint focus area “Single-Cell Approaches for Personalized Medicine,” which the BIH at Charité founded together with the Max Delbrück Center and Charité – Universitätsmedizin Berlin. The scientists are specialized in analyzing individual cells and were thus able to closely examine the patients’ blood and immune cells. “Our research showed that the pathogenic changes in the mitochondrial genome were not equally present in all cells,” explains Ludwig, a cell biologist. “For example, the mitochondria in certain types of T cells were almost completely free of mutations. That was quite surprising.”
An explanation for this finding, according to Ludwig, is that when T cells are activated, they rely on the mitochondria to supply the energy needed for their continued maturation. “During a defense response, T cells need to proliferate substantially, and we think that especially these initial cell divisions don’t work properly without healthy mitochondria.”
Selection at play
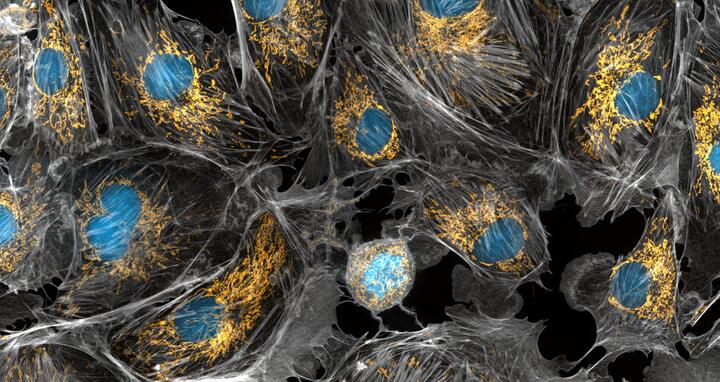
Mitochondria are the powerhouses of the cells, generating the energy the cells need to do their tasks and to stay alive. Researchers have studied mitochondria for some time because when these cell organelles don't as well as they should, several diseases develop. In this photograph of cow cells taken with a microscope, the mitochondria were stained in bright yellow to visualize them in the cell. The large blue dots are the cell nuclei and the gray web is the cytoskeleton of the cells.
Yet interestingly, different types of T cells show different degrees of tolerance to defects in the mitochondrial genome. Pathological mutations are frequently found in memory CD4+ T cells, but rarely in memory CD8+ T cells. “The way we explain this is that CD8+ T cells use the mitochondria differently,” says Ludwig. “Since they require mitochondria that are completely healthy, we only see memory CD8+ T cells without mutations. Cells with ‘sick’ mitochondria are culled out or, as we cell biologists say, negatively selected.” As this is highly relevant for patients with mitochondrial disease, the scientists now want to do further research to see exactly how the mitochondria of different cells differ.
Ludwig, whose group is based at the Berlin Institute for Medical Systems Biology of the Max Delbrück Center (MDC-BIMSB), is translating his findings into medical applications in collaboration with his clinical partners at Charité, including Prof. Lars Bullinger and Prof. Ulrich Keller, the Directors of the Department of Hematology, Oncology and Tumor Immunology at Charité Campus Virchow-Klinikum (CVK) and Charité Campus Benjamin Franklin (CBF), respectively. “We don’t yet know how therapeutically effective the use of base editing technologies – or even the transplantation of healthy mitochondria – will one day be at addressing changes in the mitochondrial genome,” says Ludwig, “but we are giving it serious thought.”
Further information
Literature
Yu-Hsin Hsieh et al. (2023): „Single-cell multi-omics of mitochondrial DNA disorders reveals dynamics of purifying selection across human immune cells“. Nature Genetics, DOI: s41588-023-01433-8
Downloads
Porträt of Leif S. Ludwig (© Felix Petermann, Max Delbrück Center)
Contacts
Dr. Stefanie Seltmann
Head of Communications
Berlin Institute of Health at Charité (BIH)
+49 (0) 30 450 543019
stefanie.seltmann@bih-charite.de
www.bihealth.org
Christina Anders
Editor, Communications
Max Delbrück Center
+49 30 9406-2118
christina.anders@mdc-berlin.de or presse@mdc-berlin.de
- About the Berlin Institute of Health (BIH) at the Charité
-
-
The mission of the Berlin Institute of Health (BIH) is medical translation: transferring biomedical research findings into novel approaches to personalized prediction, prevention, diagnostics and therapies and, conversely, using clinical observations to develop new research ideas. The aim is to deliver relevant medical benefits to patients and the population at large. As the translational research unit within Charité, the BIH is also committed to establishing a comprehensive translational ecosystem – one that places emphasis on a system-wide understanding of health and disease and that promotes change in the biomedical translational research culture. The BIH was founded in 2013 and is funded 90 percent by the Federal Ministry of Education and Research (BMBF) and 10 percent by the State of Berlin. The founding institutions, Charité – Universitätsmedizin Berlin and Max Delbrück Center for Molecular Medicine in the Helmholtz Association (MDC), were independent, member entities within the BIH until 2020. Since 2021 the BIH has been integrated into Charité as the so-called third pillar. The MDC is now the Privileged Partner of the BIH.
- Max Delbrück Center
-
The Max Delbrück Center for Molecular Medicine in the Helmholtz Association (Max Delbrück Center) is one of the world’s leading biomedical research institutions. Max Delbrück, a Berlin native, was a Nobel laureate and one of the founders of molecular biology. At the locations in Berlin-Buch and Mitte, researchers from some 70 countries study human biology – investigating the foundations of life from its most elementary building blocks to systems-wide mechanisms. By understanding what regulates or disrupts the dynamic equilibrium of a cell, an organ, or the entire body, we can prevent diseases, diagnose them earlier, and stop their progression with tailored therapies. Patients should be able to benefit as soon as possible from basic research discoveries. This is why the Max Delbrück Center supports spin-off creation and participates in collaborative networks. It works in close partnership with Charité – Universitätsmedizin Berlin in the jointly-run Experimental and Clinical Research Center (ECRC), the Berlin Institute of Health (BIH) at Charité, and the German Center for Cardiovascular Research (DZHK). Founded in 1992, the Max Delbrück Center today employs 1,800 people and is 90 percent funded by the German federal government and 10 percent by the State of Berlin.