Gene editing to treat inherited kidney disease
Approximately one in a thousand children is born with autosomal dominant polycystic kidney disease, or ADPKD, making it one of the most common genetic disorders. The condition is usually caused by a dominantly inherited mutation in the PKD1 or PKD2 gene. These mutations trigger the formation of cysts in the kidneys, which can lead to high blood pressure, pain, and infections – and, over time, to kidney failure.
“There’s currently only one approved drug for ADPKD, and it mainly treats symptoms while often causing serious side effects,” says Dr. Michael Kaminski, head of the Emmy Noether Research Group Kidney Cell Engineering at the Berlin Institute for Medical Systems Biology of the Max Delbrück Center (MDC-BIMSB). Kaminski is also a physician in the Department of Nephrology and Medical Intensive Care at Charité – Universitätsmedizin Berlin. Some patients on the drug lose up to six liters of urine per day. Additionally, it does not treat liver cysts, from which many patients also suffer. New therapies are sorely needed.
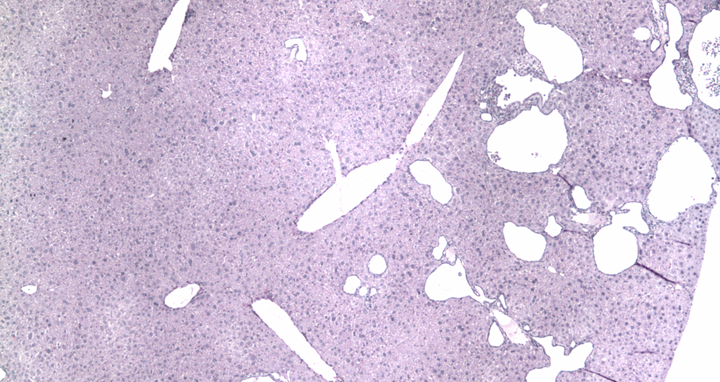
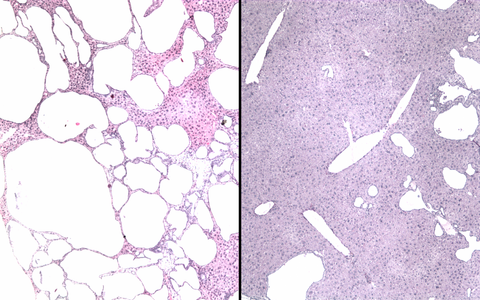
The two photos show stained tissue sections of a mouse liver before and after base editing. On the left, numerous large cysts can be seen in the animal's liver due to a genetic defect. In the right-hand image, the number and size of the fluid-filled cavities have visibly decreased as a result of the gene correction via base editing.
Now an international team led by Kaminski, Dr. Sorin Fedeles, and Dr. Matteus Krappitz report early progress in a paper published in “Molecular Therapy.” Working with colleagues from the Max Delbrück Center, Charité – Universitätsmedizin Berlin, Yale University, and the University of Colorado, the researchers successfully used base editing to correct mutations in human and mouse cells. In a mouse model, they used CRISPR/Cas mediated base editing to reduce liver cysts. The study’s co-first authors are Antonia Ibel from Kaminski’s research group and Dr. Rishi Bhardwaj from the Yale School of Medicine.
Missing protein expression restored
The researchers first focused on identifying changes in the PKD1 gene from ADPKD patients. “We found 39 different point mutations, about one-third of which we were able to correct with high precision in a cell culture system,” says Kaminski. The team then targeted a specific mutation in kidney epithelial cells collected from the urine of ADPKD patients at Charité. “In both human and mouse cells with a non-functional PKD1 gene, we were able to efficiently correct a defect that causes a shortened protein using base editing,” Kaminski explains. In treated mouse cells, expression of the protein polycystin-1– encoded by the PKD1 gene – was restored, and levels of a cellular stress marker declined.
Finally, the researchers tested their approach in a mouse model of ADPKD. They packaged the components for base editing into adeno-associated viruses and delivered them to the mice. “We saw that the base editor worked particularly well in liver cells – both the number and size of cysts decreased significantly,” says Kaminski. “This could be especially useful for patients because the only approved drug for ADPKD, Tolvaptan, doesn’t reduce liver cysts,” he adds.
Unclear if gene therapy works in later disease stages
Nevertheless, Kaminski is not yet satisfied with the results. “We’re currently developing delivery systems to get the gene-editing tools more effectively into the kidney – right at the core of the problem,” he says. He and his team also plan to target other ADPKD-causing mutations and to investigate the effects of gene correction. Another open question is the best timing for treatment. “In our current model, we applied base correction very early in the disease process,” Kaminski says. “I’d like to find out whether the method could also reduce cysts in more advanced stages of disease.”
Text: Anke Brodmerkel
Further information
Literature
Antonia Ibel, Rishi Bhardwaj, et al. (2025): “In vivo base editing reduces liver cysts in autosomal dominant polycystic kidney disease.” Molecular Therapy, DOI: 10.1016/j.ymthe.2025.08.026
Contact
Dr. Michael Kaminski
Group leader, Kidney cell Engineering
Berliner Institute for Medical Systems Biology of the Max Delbrück Center (MDC-BIMSB)
+49 30 94060-1372
michael.kaminski@mdc-berlin.de
Jana Schlütter
Editor and Deputy Head
Max Delbrück Center
+49 30 9406-2121
jana.schluetter@mdc-berlin.de oder presse@mdc-berlin.de
- Max Delbrück Center
The Max Delbrück Center for Molecular Medicine in the Helmholtz Association lays the foundation for the medicine of tomorrow through our discoveries of today. At locations in Berlin-Buch, Berlin-Mitte, Heidelberg, and Mannheim, interdisciplinary teams investigate the complexity of disease at the systems level – from molecules and cells to organs and entire organisms. Together with academic, clinical, and industry partners, and as part of global networks, we turn biological insights into innovations for early detection, personalized therapies, and disease prevention. Founded in 1992, the Max Delbrück Center is home to a vibrant, international research community of around 1,800 people from over 70 countries. We are 90 percent funded by the German federal government and 10 percent by the state of Berlin.