Ancient viral DNA shapes modern human placentas
Joint press release by Max Delbrück Center and University of Bath
The human genome is riddled with relics of viral infections – bits of DNA from viruses that have been inserted in human DNA over millions of years and never left. Most are silent but some have taken on functional roles, particularly in organs that evolve relatively rapidly, including the placenta.
An international collaboration between geneticists, evolutionary biologists, bioinformaticians and clinicians has identified how some of these ancient viral DNA fragments are influencing new life today – specifically, helping regulate genes that control normal placenta development and operation. One particular gene, EPS8L1, when overexpressed, induces key features of pre-eclampsia, a potentially life-threatening disorder during pregnancy. The research was reported this week in “Genome Biology.”
“These findings connect a deep evolutionary process to a very modern clinical problem and point to a potential biomarker to detect pre-eclampsia risk before symptoms develop,” says Professor Zsuzsanna Izsvák, Group Leader of the Mobile DNA Lab at the Max Delbrück Center in Berlin and co-corresponding author.
About 5% of pregnancies are affected by pre-eclampsia, which can be extremely dangerous for both mother and fetus. There is no cure and the most serious cases require early delivery. The precise cause of the disorder remains elusive in part because it is so difficult to study.
AI to the rescue
Using A100 Beast — a deep-learning model developed by Dr. Amit Pande in Izsvák’s lab — the researchers classified DNA sequences that regulate gene expression across species. “We taught the AI to read DNA as language,” says Pande. “It predicted previously overlooked enhancer regions, many of viral origin, giving us the first clues.” In placental genomes, A100 Beast pinpointed a cluster of highly active ERV3-MLT1 enhancers.
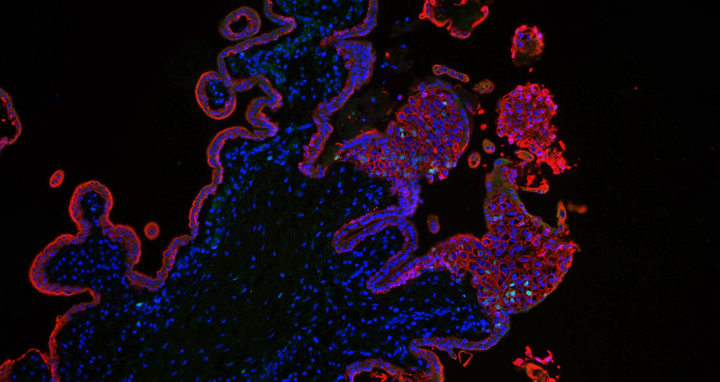
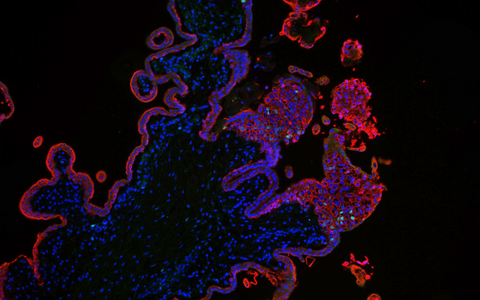
The stained cells are trophoblasts of the placenta tissue.
The lab partnered with University of Bath, and clinical centers including the Blois lab at Universitätsklinikum Hamburg-Eppendorf the Müller / Dechend lab at the Experimental and Clinical Research Center in Berlin-Buch, as well as others in Essen, Würzburg and Oslo to analyze placental tissue from both healthy pregnancies and pre-eclampsia pregnancies. The studies confirmed 87 virus-derived enhancers active in the placenta and that the enhancers help boost activity of nine genes commonly dysregulated in pre-eclampsia.
“We were surprised,” says co-first author Dr. Manvendra Singh, who did this work at the Izsvak lab and is now a group leader at INSERM Paris, “because we have DNA from dozens of viral families in our genome, but it was just one particular family, ERV3-MLT1, linked with pre-eclampsia.”
Dr. Rabia Anwar in the lab.
Among the commonly dysregulated genes, the team became interested in one that had not been studied before: EPS8L1. The gene is expressed in trophoblasts, the critical cells that form the outer layer of the blastocyte in the first days of pregnancy and become the placenta.
Functional studies, designed and conducted by co-first author Dr. Rabia Anwar at the Izsvak lab, demonstrated that when EPS8L1 is overexpressed in placental cell cultures, the cells show signs of pre-eclampsia, including reduced trophoblast invasion capacity, altered blood vessel formation and oxidative stress and tissue damage in the placental cells. However, complete knockout of the gene led to cell death, suggesting it is required for normal function.
Potential biomarker
The team also found that a secreted form of EPS8L1 was detected in maternal blood, where its levels correlated with established pre-eclampsia biomarkers. This means it could potentially be used in blood screening panels for early-onset pre-eclampsia long before dangerous symptoms appear.
Notably, the EPS8L1 gene was consistently upregulated across all the cohorts providing tissue samples.
“This was exciting because you want a biomarker to be present across a wide a variety of ethnic backgrounds to be as useful as possible,” says Rabia Anwar, who is now a postdoctoral researcher at University Health network in Toronto. “We also found that the gene was not associated with a different pregnancy complication, another indication it could work quite well for pre-eclampsia specifically.”
A larger clinical study is needed to confirm that the EPS8L1 protein could be used as a biomarker to detect pre-eclampsia risk in the first trimester of pregnancy.
100 million years ago
Beyond its medical relevance, the study illustrates how ancient viruses continue to shape human biology. The viral DNA that is the focus of this study was introduced to primates more than 100 million years ago before splitting from rodents in the evolutionary tree – something the researchers can confirm because it is present in a common mammalian ancestor that primates share with rodents.
“It’s a reminder that there is much more to learn about our genome and how ancient infections can influence who we are today,” adds Professor Laurence D. Hurst, an expert for evolutionary genetics at the University of Bath and co-corresponding author.
The deep-learning framework A100 Beast is freely available on Hugging Face Spaces, allowing other scientists to explore viral and non-viral enhancers across species.
Text: Laura Petersen
Further information
Literature
Rabia Anwar, Amit Pande, Manvendra Singh, et al. (2025): „ERV3-MLT1 provides cis-regulatory elements for human placental functioning and are commonly dysregulated in human-specific pre-eclampsia”. Genome Biology 26, 364. DOI: 10.1186/s13059-025-03821-1
Pictures to download
- Dr. Rabia Anwar in the lab. Photo: Mariam Parashos
- The stained cells are trophoblasts of the placenta tissue. Foto: Rabia Anwar
Contacts
Jana Schlütter
Editor and Deputy Head, Communication
Max Delbrück Center
+49 30 9406-2121
jana.schluetter@mdc-berlin.de or presse@mdc-berlin.de
Vicky Just
Media and PR Manager
University of Bath, UK
+44 07966 341357
vjj21@bath.ac.uk or press@bath.ac.uk
- Max Delbrück Center
The Max Delbrück Center for Molecular Medicine in the Helmholtz Association lays the foundation for the medicine of tomorrow through our discoveries of today. At locations in Berlin-Buch, Berlin-Mitte, Heidelberg, and Mannheim, interdisciplinary teams investigate the complexity of disease at the systems level – from molecules and cells to organs and entire organisms. Together with academic, clinical, and industry partners, and as part of global networks, we turn biological insights into innovations for early detection, personalized therapies, and disease prevention. Founded in 1992, the Max Delbrück Center is home to a vibrant, international research community of around 1,800 people from over 70 countries. We are 90 percent funded by the German federal government and 10 percent by the state of Berlin.
- University of Bath
The University of Bath is one of the UK's leading universities, recognized for high-impact research, excellence in education, an outstanding student experience and strong graduate prospects.
- We are ranked among the top 10% of universities globally, placing 132nd in the QS World University Rankings 2026.
- We are ranked in the top 10 in all of the UK’s major university guides.
- The University achieved a triple Gold award in the last Teaching Excellence Framework 2023, the highest awards possible, for both the overall assessment and for student outcomes and student experience. The Teaching Excellence Framework (TEF) is a national scheme run by the Office for Students (OfS).
- We are The Times and The Sunday Times Sport University of the Year 2026.
Research at Bath is shaping a better future through innovation in sustainability, health, and digital technologies.