Myxovirus-resistance proteins
Myxovirus-resistance proteins
Mx proteins are interferon-induced GTPases and central players of the innate immune system. They mediate cellular resistance against a wide range of pathogens, including influenza viruses and many other related viruses. As typical members of the dynamin superfamily, they tubulate liposomes and oligomerize in ring-like structures around these membrane tubules. However, they can also directly interact with viral particles in infected cells leading to their sequestration.
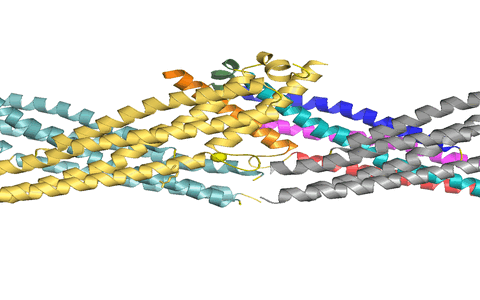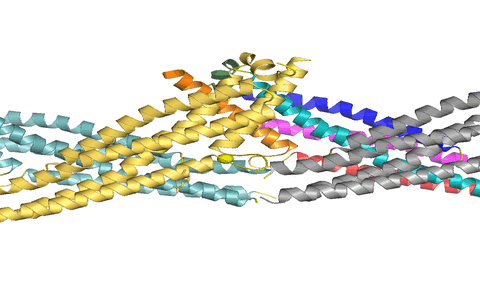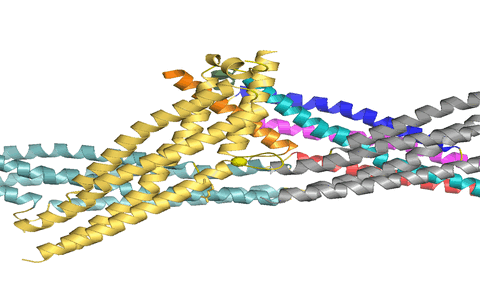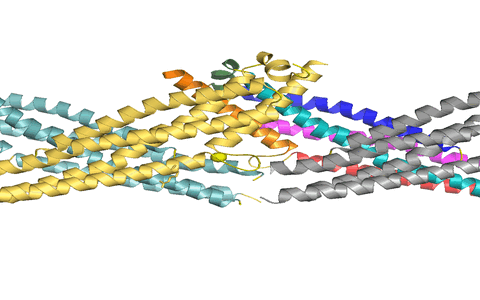
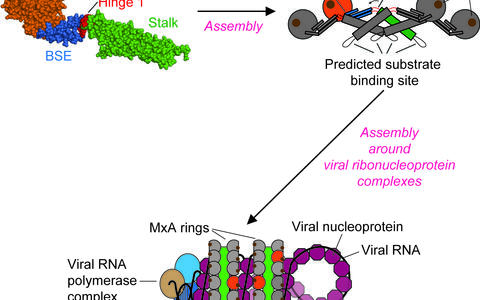
Using X-ray crystallography, we determined the structure of the virus binding domain, the stalk, of human MxA which folds into a four-helical bundle. In the crystals, the stalks assembled via three distinct interfaces in a zigzag fashion. We showed by biochemical and antiviral experiments that all three interfaces are required for the assembly of full-length MxA and for the antiviral activity. Based on these results, we suggested a model for ring-like oligomers of MxA and a mechanism for the activation of GTPases activity by dimerization of GTPase domains of neighbouring MxA rings.
We also determined the full-length MxA structure. We found a third domain, the bundle signalling element (BSE), in the center of the MxA molecule which bridges GTPase domain and stalk. Interestingly, the BSE contacted the GTPase domain of the same molecule but was only loosely associated with the stalk. However, the BSE formed an interface with the stalk of the neighbouring molecule, and this interface was important for assembly and the antiviral function. This suggested a pathway how nucleotide-induced changes in the oligomer are transmitted from the GTPase domain to the stalk.
Publications:
Gao S., von der Malsburg A., Paeschke S., Behlke J., Haller O. , Kochs G., Daumke O. (2010) Structural basis of oligomerization in the stalk region of dynamin-like MxA. Nature 465, 502-506.
Gao S., von der Malsburg A., Dick A., Faelber K., Schröder G.F., Haller O., Kochs G., Daumke O. (2011) Structure of the interferon-induced MxA GTPase sheds light on the domain interplay during the antiviral function. Immunity 35, 514-25.
Researchers in my group
Song Gao (PhD 2011), Daniel Olal (PostDoc), Alexej Dick (PhD student)
Collaborators
Otto Haller and Georg Kochs, University of Freiburg.
Figure 1: MxA stalks assemble via interface-2 to form stable stalk dimers. We suggested that these dimers can rotate around interfaces-1 (top) and -3 (bottom) to allow formation of helical filaments.
Figure 2: Model for MxA action. MxA monomers assemble in the cytosol into stable MxA tetramers. These tetramers can further assemble around viral ribonucleoproteins therefore interfering with replication of the virus.