Höpken Lab
Microenvironmental Regulation in Autoimmunity and Cancer
Profile
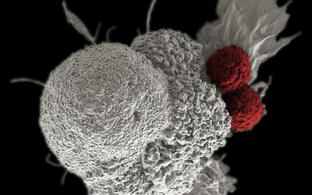
Our group elucidates tumor-stroma interactions in hematological diseases and defines growth- and survival niches for B cell leukemia/lymphoma. By combining pre-clinical mouse models, in vivo imaging, cellular and molecular biology techniques as well as gene expression profiling substantial insight into the complex and dynamic network of tumor cells and the benign infrastructure within lymphatic tissues has been obtained. Targeting the stromal environment is an interesting therapeutic option because this benign compartment is subject to low selective pressure for genetic mutations compared with the tumor cells themselves.
Secondly, we developed Chimeric Antigen Receptor (CAR) T cells for the treatment of B cell disorders, such as a neoplastic diseases of plasma cells and mature B cells, in particular multiple myeloma (MM) or non-Hodgkin’s lymphomas (B-NHLs). Recent demonstration of disease regression of lymphoma by CD19-specific T cells has vitalized adoptive T cell therapy in the scientific community. We already succeeded to develop CAR-T cells that will allow treatment of lymphoid tumors that carry B cell differentation antigens. Examples include i) anti-tumor activity in the treatment of MM, ii) anti-plasma cell activity in dysregulated B cell responses during autoimmunity, and iii) anti-tumor activity in the treatment of mature B-NHLs.
Team
Research
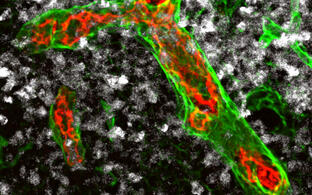
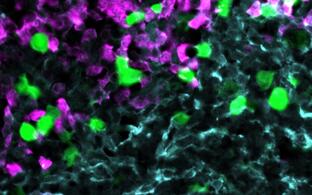
Our group studies the crosstalk between benign and malignant lymphocytes with stromal cells including mesenchymal cells and innate immune cells within a cancerous or autoimmune microenvironment. We observed an intimate interaction between immune and stromal cells during chronic inflammation, but also for lymphoma stroma in which an inflammatory milieu and tissue remodeling promotes tumor progression. In particular, innate immune cells, such as monocytes, neutrophils and dendritic cells play an import regulatory role within the lymphoid tumor microenvironment and are the focus of our research. Targeting the stromal environment is an appealing treatment option for lymphoma because this benign compartment is subject to low selective pressure for genetic mutations compared with the tumor cells themselves.
Secondly, we develop Chimeric Antigen Receptor (CAR) T cells for the treatment of B cell disorders, such as a neoplastic diseases of plasma cells, memory B cell and/or mature B cells, in particular multiple myeloma (MM) or non-Hodgkin’s lymphomas (B-NHLs), as well as for autoimmune diseases.
Recent work from my group on dissecting tumor microenvironment (TME)-imposed mechanisms of immune evasion in lymphoma and leukemia impacts on our current development of next generation CAR T cells that will be able to overcome stromal barriers and an immunosuppressive microenvironment.
Cellular immune therapies utilizing adoptive transfer of CAR- or TCR-engineered T cells are promising novel treatment option for a broad range of cancers. However, the timely translation from basic and pre-clinical research of these complex products toward clinical trials poses many challenges. To overcome major hurdles, a detailed knowledge of the immune system including the elucidation of general principles of T cell biology, the exploration of an inhibitory TME, and the identification of novel suitable target antigens, is warranted.
My group in close collaboration with Armin Rehms group (MDC) developed CAR T cells for the treatment of B cell disorders, such as a neoplastic diseases of plasma cells, memory B cell and/or mature B cells, in particular MM or B-NHLs. We entered the translational phase by setting up a GMP compliant automated and closed CAR T cell manufacturing process (CliniMACS Prodigy) and by establishing regulatory affairs for our present CARs. A proof-of-concept BCMA-CART trial application has been granted and the trial is currently prepared together with clinical partners from the NCT/Dresden and the NCT/Heidelberg.
Mechanistical aspects such as cell biological regulation of T effector cell function and their capacity to traffic and interact to and within the TME will be instrumental to manipulate differentiation and functionality of immune cells for therapeutic intervention.
Hence, our research program on tumor immunotherapy integrates the insights into cell biological regulation of effector T cell function with the pre-clinically and clinically validation of various CARs directed against hematologic neoplasia and beyond. We are currently generating CARs which have advantages foremost in their
- capacity to eradicate hematopoietic tumors at low effector to target ratios,
- functionality in an allogeneic setting, and in their
- capacity to traffic and function to and within sites where tumor cells preferentially home to.
Novel imaging approaches will fill the gap between tumor and stroma cell phenotypes, stroma cell remodeling analyzed by static morphological methods, and the dynamic benign and malignant lymphocyte behavior in a living animal has already been implemented in my group.
Future Directions
During the next years, we aim to identify novel T cell antigens in different cancer entities and we will develop strategies to improve CAR T cell efficiency with the goal to overcome stromal barriers and the immunosuppressive microenvironment as well as exhaustion of therapeutic T cells. Additionally, we aim to visualize the kinetics of CAR T cell function in particular their killing capacities. For that we will employ two-photon and light sheet microscopy to quantify the spatiotemporal dynamics of CAR T cell-mediated killing in appropriate mouse models or in human 3-D cell culture systems. Collectively, our ongoing and future research on CAR T cell therapy will improve the basic-translational interface to advance personalized treatment of cancer patients.
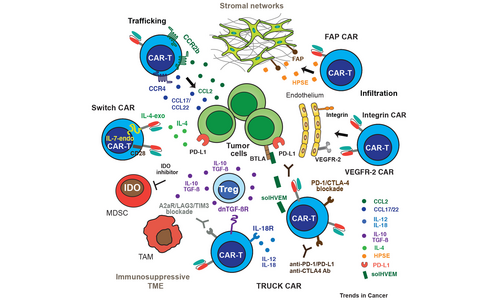
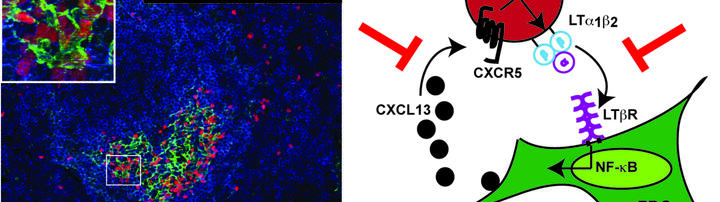
Figure 1: Strategies of next generation CAR-T cells to overcome the hostile tumor microenvironment.
Trafficking of CAR-T cells to the tumor niche is facilitated mainly through expression of chemokine receptors (CCR4, CCR2b) by the CAR-T cells and expression of their respective ligands, chemokines, by the vasculature and by stromal cell networks. CAR-T cells expressing HPSE (heparanase), or targeting FAP, integrin, and VEGFR-2 foster infiltration in stroma and vasculature. TRUCK CAR-T cells secrete cytokines like IL-12 or IL-18, solHVEM, or dn TGF-ßR to overcome checkpoint inhibition. Blockage of immune checkpoint inhibition, i.e. A2aR (adenosine receptor), LAG3, TIM3, PD1, and CTLA4 can be facilitated by anti-immune checkpoint drugs or by TRUCK CARs co-expressing anti-PD1/anti-CTLA4/anti-PD-L1 antibodies. Within the TME TAMs and MDSCs secrete IDO and adenosine. Switch receptor CAR-T cells convert a dominant negative signal to positive ones, i.e. IL-4 binding ectodomain fused to IL-7 signaling endodomain or PD-1 extracellular with CD28 endodomain.
Funding
Our work is kindly supported by the Deutsche José-Carreras-Leukämiestiftung, the Deutsche Krebshilfe, the Berliner Krebshilfe, the Helmholtz-Validierungsfond, the Bundesministerium für Bildung und Forschung (BMBF/VIP+) and the Impuls- und Vernetzungsfonds der Helmholtz-Gemeinschaft “Zukunftsthema Immunology und Inflammation“.