Model partnership with NCT Berlin
The National Center for Tumor Diseases (NCT,) a long-term collaboration between the German Cancer Research Center (DKFZ), university hospitals, and other research partners, is supporting a promising new therapy for patients with advanced solid tumors. Developed by Captain T Cell, the TCR-T cell therapy will be studied in a multi-center clinical trial in 2027. The trial will be led by Dr. Antonia Busse, a senior physician in the Department of Hematology, Oncology, and Tumor Immunology at Charité’s Benjamin Franklin campus. Charité is also the study sponsor and holds legal responsibility for its execution.
Advanced and hard-to-treat solid tumors are among the world’s leading causes of death –second only to cardiovascular disease. While CAR-T cell therapies have already shown major success in treating blood cancers, similar immunotherapies have so far proven far less effective against solid tumors, such as those in the lung, bladder, soft tissue, or head and neck.
The ToMA4TA1 trial will evaluate the TCR-T therapy in up to 24 patients with advanced solid tumors. It aims to assess the treatment’s safety, optimal dosing, and initial signs of efficacy. The study will be coordinated by NCT Berlin, with additional participation from NCT sites in Dresden, Heidelberg, SouthWest, WERA, and West, along with the Nuremberg Hospital as an external partner. Decades of foundational research at the Max Delbrück Center and the Berlin Institute of Health at Charité (BIH) ¬– supported by Germany’s Federal Ministry of Research, Technology, and Space – have laid the groundwork for this trial.
A Milestone for NCT Berlin
The study is a joint initiative of the biotech start-up Captain T Cell, a successful spin-off from the Max Delbrück Center, and Charité. “This funding marks an important step forward for personalized cancer therapy – and a milestone in the development of NCT Berlin, which is leading the scientific effort and gaining momentum in its research activities,” says Professor Ulrich Keilholz, Spokesperson and Managing Director of NCT Berlin. He describes the collaboration between Captain T Cell researchers and top university cancer centers across Germany as “a model for how the NCT is driving high-level cancer research through nationwide cooperation.”
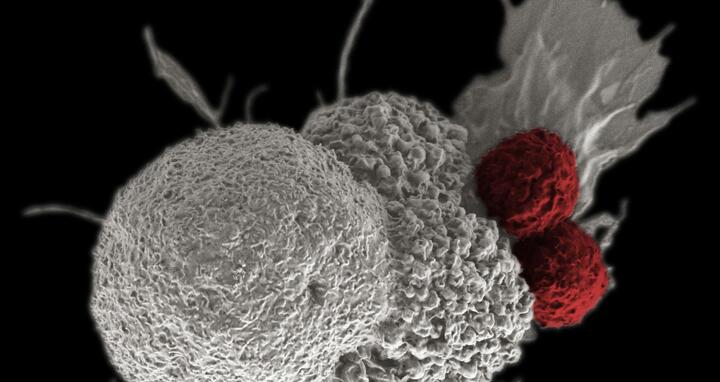
TCR-T cell therapy uses T cells equipped with a genetically engineered receptor, or TCR, that can recognize a specific protein found in several types of solid tumors: the MAGE-A4 tumor antigen. These engineered T cells can detect not just surface markers but also hidden structures inside cancer cells. They’re also molecularly optimized to survive and stay active longer in the hostile tumor microenvironment.
A key feature of the ToMA4TA1 study is that it will – for the first time – target patients with the HLA-A1 tissue marker. By contrast, the first TCR-T cell therapy approved in the U.S., which also targets MAGE-A4, is only suitable for patients with the HLA-A2 marker –excluding many potential candidates. The new study opens door to patients previously left without options.
New hope for patients
“In clinical practice, unfortunately we often see people who have exhausted all conventional treatment options and urgently need alternatives,” says Busse. “Innovative TCR-T therapies could offer meaningful treatment advances – even for stages of disease once considered untreatable.”
“The preclinical data are promising and give us reason to believe this therapy can make a real impact,” adds Dr. Felix Lorenz, CEO and CSO of Captain T Cell. “It’s a significant moment for our team to see our TCR-T cell therapy move into clinical testing. Our goal is to give patients with no remaining options a new path forward.”
Patients involved from the start
“I don’t need to understand the technical workings of antigens. My role is to assess whether and how patients might benefit from this therapy,” says Ulla Ohlms, spokesperson for the NCT Berlin Patient Research Council who has been part of the study’s planning from the beginning as a patient representative. To her, “the ToMA4TA1 study is a clear proof of concept. It’s about exploring new therapeutic possibilities to help people keep living when everything else has failed. That’s what I call real benefit.”
Text: Sandra Giannakoulis-Markus, NCT Berlin
Weiterführende Informationen
- Captain T Cell secures seed financing round
- New funding for Captain T Cell
- A long and winding road against cancer
- National Center for Tumor Diseases
The National Center for Tumor Diseases (NCT) is a long-term cooperation between the German Cancer Research Center (DKFZ), excellent partners in university medicine and other outstanding research partners at various locations in Germany.
Heidelberg was the first NCT site opened in 2004, Dresden became operational in 2015. On February 2, 2023, the BMBF announced new sites: Berlin, SüdWest (Tübingen/Stuttgart-Ulm), WERA (Würzburg with partners Erlangen, Regensburg and Augsburg) and West (Cologne/Essen). Together, the six NCT sites are now cooperating with the DKFZ to advance clinical cancer research in Germany and to improve treatment outcomes and the quality of life of cancer patients. At NCT Berlin, researchers from Charité – Universitätsmedizin Berlin, the Berlin Institute of Health at Charité (BIH), and the Max Delbrück Center work closely together.The goal of the NCT is to rapidly and strategically translate innovations in cancer research into clinical trials in Germany – enabling state-of-the-art cancer diagnosis and treatment while maintaining a high quality of life for patients.