The tissue engineer
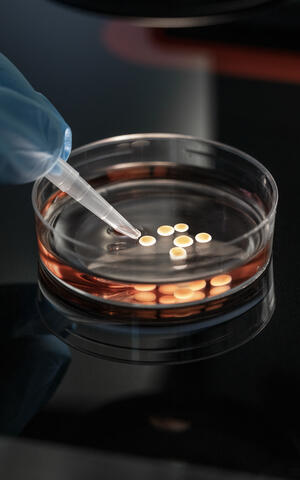
Dr. Mina Gouti cradles the petri dish in her hand so that the tiny white spheres move around in the pink liquid. She casts a protective glance at them. Her colleague brought the organoids to life a month ago, and since then they have grown to just over a millimeter. “In the beginning they are given nutrients every day to ensure they grow and thrive, and later on every two to three days,” Gouti says, carefully placing the dish with organoids back into the incubator, which keeps the temperature at a comfortable 37 degrees Celsius, just like in the human body. “If we take good care of them, they can grow to six millimeters in size and live up to two years.”
Organoids can live up to two years in the Petri dish.
The organoids Gouti creates in the lab are organ-like tissue structures made of spinal cord nerve cells and associated muscle cells. It’s easy to tell the difference between the two – the dark core of muscle and the lighter rim of surrounding nerve tissue – when looking at them under a microscope. The tiny bundles of muscle twitch rhythmically. “They contract like muscles in the human body,” says Gouti, who heads the Stem Cell Modeling of Development and Disease Lab at the Max Delbrück Center for Molecular Medicine in the Helmholtz Association (MDC). Her enthusiasm is infectious. “This is fascinating because it shows that we have generated functional tissue.”
She is developing the organoids from reprogrammed stem cells from patients suffering from neuromuscular diseases like spinal muscular atrophy (SMA). The fatal disease causes the motor neurons responsible for bodily movements to eventually die. “The children experience paralysis in the first months of life, and in the end they can’t even breathe,” Gouti says. “With the help of these organoids, we want to understand why exactly the motor neurons die and find ways to stop this process.” Gouti is also looking to organoids to shed light on other currently incurable neuromuscular diseases such as amyotrophic lateral sclerosis (ALS), which affects adults.
“I fell in love with stem cells”
Mina Gouti is a developmental biologist. From an early age she was interested in how the human body becomes what it is: “At school I asked so many questions that my biology teacher advised me to become a scientist and answer them all myself.” She left her hometown of Athens to study molecular biology in London. In the lab she saw postdocs conduct research on cell cultures derived from mouse embryonic stem cells. “The stem cells were off limits for me at the beginning,” Gouti recalls. “But when I finally got to work with them, I was totally excited and instantly fell in love with them because of their unlimited potential. If you want something from stem cells, you have to learn to control them.”
The MDC’s Pre-GoBio program is funding Gouti’s idea of using bioreactors to produce organoids on a larger scale. These are large enough to cultivate 200 of the white spheres in liquid nutrients, where they can grow older than in petri dishes.
She mastered all the necessary techniques and studied what signaling pathways cause stem cells to differentiate into central nervous system cell types as part of her doctoral thesis. Just two years earlier Shinya Yamanaka of Japan had produced the first induced pluripotent stem cells (iPSCs), ushering in the ability to reverse cell fates: All of a sudden it was possible to reprogram a skin cell into a stem cell, from which in turn any other cell in the body may be created.
Now Gouti was generating neurons of the brain and cervical spine from pluripotent stem cells. But she hit a roadblock with respect to neurons of the lower spinal cord. At the Francis Crick Institute in London she became aware of neuromesodermal progenitors (NMPs), a transient cell population first discovered in 1884 that can differentiate into neurons and muscle cells. Were they the crucial precursor for all other cells of the spinal cord? In May 2013 she succeeded in producing in the laboratory the first NMPs from pluripotent stem cells, which were also capable of forming neurons of the lower spinal cord and the associated skeletal muscle.
Three surprises
At the MDC, which she joined in 2016 as a junior group leader, Gouti bet everything on the NMP card. And rightly so, as it soon turned out. Her lab focused on pluripotent stem cell derived NMPs that resemble those in the human body. “Shortly thereafter I had the bold idea of growing them into neuromuscular organoids and seeing if they would actually form both cell types – neurons and muscle cells in 3D – just like in the human body,” Gouti says. No one had been able to do that before.
Those are the moments you live for as a scientist – to see something completely unexpected, but that makes 100 percent sense.
She created the perfect environment, placing the NMP cells and nutrient solution into the wells of a non-adherent microwell plate, and watched as small, intact spheres emerged. “After five days we saw through the microscope that both tissue types were indeed forming.” It was the first of three surprises. The 3D cultures were so robust that they stayed alive for a month or more. On day 40 Gouti and her graduate student Jorge Martins noticed that the organoids were contracting. This meant that the motor neurons were extending their axons to the muscle cells and causing them to move through a synapse called the neuromuscular junction or endplate. The spinal cord and muscles were exchanging signals. What a sensation!
They also discovered that in the neuromuscular organoids (NMOs) Schwann cells and an advanced neural network had formed, and that these had assumed the function of central pattern generator (CMG) like circuits – i.e., they were sending out rhythmic signals such as those needed for breathing and walking. This was another sensation. Her high-profile paper appeared in Cell Stem Cell in 2020. “For the first time we were able to observe such a complex network in a human model,” Gouti says. “Those are the moments you live for as a scientist – to see something completely unexpected, but that makes 100 percent sense.”
Can organoids grow old?
The successes confirmed her hope that NMOs could help in the search for targeted and timely therapies for neuromuscular diseases. She now wants to culture position-specific organoids that correspond to particular spinal cord segments and their associated muscles. And she wants to coax these organoids to develop blood vessels, so they can grow larger and older to more closely resemble adult tissue. “We could then track exactly when and where an adult disease like amyotrophic lateral sclerosis appears in the tissue and whether other cell types are affected before motor neurons die and paralysis ensues,” Gouti says. “We could try to save these cells before it’s too late.” NMOs are also opening up new avenues to study neurodegenerative diseases.
Mina Gouti at a sterile hood. She replaces the nutrient fluid for the organoids with a pipette.
Gouti’s long-term goal is to create all central nervous system cell types from stem cells and develop an organoid that can transmit neural signals from the brain along the entire spinal cord all the way to the muscles. She wants to observe where and how communication goes wrong when people get sick. In 2020 she received a European Research Council (ERC) Consolidator Grant to pursue these new endeavors.
“Organoids also help us reduce animal research leading up to clinical trials,” Gouti says. “Unfortunately, we can’t stop doing such research completely. We need more complex models that better mimic human physiology.”
Stained neurons and muscle cells become true works of art
On this afternoon, her team is setting up a high-throughput imaging system one floor above the organoid lab. A robotic arm pipettes hundreds of different drugs and tests them on patient-specific organoids to see if they are effective for treating spinal muscular atrophy. The first images suddenly appear on the screen. These stained muscle cells and neurons with long axons – microstructures exploding in greens, yellows, reds and blues – are veritable works of art. “The resolution is incredible!” Gouti enthuses. She can’t wait to take full advantage of the technology’s new capabilities.
For all her enthusiasm for the workings of motor neurons, stem cells, and organoids, Gouti is also enamored by their beauty. She has published in Greece a book of images of her lab work and displayed them in two exhibitions. She wants to bring stem cell research closer to the public. For each picture she interviewed writers and children with cancer. “In the red-stained astrocytes that were generated from stem cells, the writer saw dancing cells and the girl a tunnel leading to an unknown destination,” Gouti says. “I very much hope that we can use organoids to find ways to better help people with fatal neuromuscular diseases.”
Text: Mirco Lomoth
Further information