What statins do to muscles
Around 20 million people worldwide take statins. The number tops nearly five million in Germany alone. The drugs are prescribed to lower cholesterol levels and prevent secondary diseases such as heart attacks and strokes. “However, statins are also associated with a number of side effects, which is why many patients do not reliably take them as prescribed,” says Professor Simone Spuler, head of the MDC’s Myology Research Group and the Muscle Research Unit at the Experimental and Clinical Research Center (ECRC), a joint institution of the MDC and Charité – University Medicine Berlin.
The most common adverse side effects of statins include muscle pain and cramps. “But given the benefits of statins for the health of the Western world’s population, these side effects are often considered negligible,” says Spuler. She and her team wanted to gain a more complete picture and find out what statins actually trigger in the muscle cells. To this end, they initiated – with DFG funding alone and without the support of the pharmaceutical industry – a study that has now been published in the journal Scientific Reports.
Statins disrupt the production of more than 900 proteins
It is quite obvious that normal amounts of statins applied as active substances exert dramatic structural, functional and metabolic effects on the muscles.
In their study, the group under the study’s lead author Dr. Stefanie Anke Grunwald from the ECRC Muscle Research Unit exposed a total of 22 populations of human skeletal muscle cells to two different statins each: the liposoluble substance simvastatin and the hydrosoluble substance rosuvastatin. The researchers then investigated which genes in the cells were activated and converted into proteins and which were not. They also analyzed the cells’ metabolism and assessed their condition on the basis of morphological criteria.
“The task of the statins is to block a specific enzyme in cholesterol formation, HMG-CoA,” Grunwald explains. There have been several studies in the past that shed light on the effects of statins on human muscle. “Yet many of these studies were not conducted on muscle cells or human cells,” says Grunwald. The elimination of a central enzyme can have complex consequences – which she and her colleagues have now illuminated using state-of-the-art computer modeling methods.
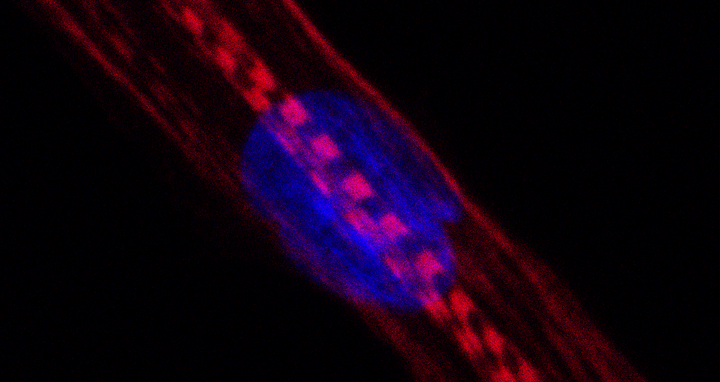
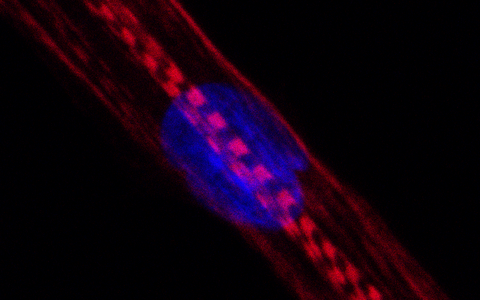
Human muscle cell. In people taking statins, the production of myosins, among other things, was disturbed. These proteins are stained red in the picture, the cell nucleus blue. The horizontal stripes show that the cell is developing into a mature muscle fibre.
“The results were highly interesting,” says Spuler. “It is quite obvious that normal amounts of statins applied as active substances exert dramatic structural, functional and metabolic effects on the muscles.” For example, she and her team discovered some 2,500 genes in the cells under investigation that were regulated differently than usual when interacting with the medication. This altered the production of more than 900 proteins. They were produced in quantities which were either too small or too large. In this respect, the influence of simvastatin was greater than that of rosuvastatin.
Cells did not grow as usual
Both statins slowed not only the biosynthesis of cholesterol in the muscle cells, but also the general fatty acid metabolism and the production of eicosanoids. This is a group of hormone-like substances formed from polyunsaturated fatty acids. They act both inside and outside cells as signaling molecules and are integrated into numerous biological mechanisms. Among other things, they are involved in the development of differentiated muscle cells from muscle progenitor cells. “And they are also involved in the mechanisms by which pain develops. This was an important indication for us that we are on the right track,” says Grunwald.
“Using functional analyses, we were able to confirm that the development, growth and division of skeletal muscle cells are affected by the statins,” says Spuler. She and her team found a way to slightly reduce the negative effects of the drugs: “The administration of omega-3 or omega-6 fatty acids partially reversed the effects of simvastatin and rosuvastatin,” says the scientist. Supplementary intake of such preparations could therefore be a way to prevent or treat statin myopathy.
Statins are not lifestyle pills
“Nevertheless, I believe that our findings should lead to a much more critical view of the administration of statins in the future than is currently the case,” says Spuler. In many Western countries, cholesterol reducers have almost become lifestyle drugs. “This is by no means a positive trend,” says Spuler. In her opinion, doctors and patients should carefully weigh the benefits and potential risks of the medications in each individual case
Text: Anke Brodmerkel
Further information
Literature
Grunwald, Stefanie Anke et al. (2020): „Statin-induced myopathic changes in primary human muscle cells and reversal by a prostaglandin F2 alpha analogue”, Scientific Reports, DOI: 10.1038/s41598-020-58668-2.
Downloadable picture
Human muscle cell. In people taking statins, the production of myosins, among other things, was disturbed. These proteins are stained red in the picture, the cell nucleus blue. The horizontal stripes show that the cell is developing into a mature muscle fibre. Foto: Andreas Marg, MDC.
Contacts
Prof. Simone Spuler
Experimental and Clinical Research Center (ECRC)
Max Delbrück Center for Molecular Medicine in the Helmholtz Association (MDC) and Charité – Universitätsmedizin Berlin
Head of the Myology Research Group
+49 (0)30 450 5405 01 or +49 (0)30 450 5405 04
simone.spuler@mdc-berlin.de or simone.spuler@charite.de
Jana Schlütter
Max Delbrück Center for Molecular Medicine in the Helmholtz Association (MDC)
Editor, Communications Department
+49 (0)30 9406 2118
jana.schluetter@mdc-berlin.de or presse@mdc-berlin.de
- The Max Delbrück Center for Molecular Medicine (MDC)
-
The Max Delbrück Center for Molecular Medicine in the Helmholtz Association (MDC) is one of the world’s leading biomedical research institutions. Max Delbrück, a Berlin native, was a Nobel laureate and one of the founders of molecular biology. At the MDC’s locations in Berlin-Buch and Mitte, researchers from some 60 countries analyze the human system – investigating the biological foundations of life from its most elementary building blocks to systems-wide mechanisms. By understanding what regulates or disrupts the dynamic equilibrium in a cell, an organ, or the entire body, we can prevent diseases, diagnose them earlier, and stop their progression with tailored therapies. Patients should benefit as soon as possible from basic research discoveries. The MDC therefore supports spin-off creation and participates in collaborative networks. It works in close partnership with Charité – Universitätsmedizin Berlin in the jointly run Experimental and Clinical Research Center (ECRC), the Berlin Institute of Health (BIH) at Charité, and the German Center for Cardiovascular Research (DZHK). Founded in 1992, the MDC today employs 1,600 people and is funded 90 percent by the German federal government and 10 percent by the State of Berlin.