Myologie
Profile
However, for persons with genetic muscle diseases, this happy state-of-affairs is not the case.
These persons are wheel chair bound from child- or young adulthood until they die. There are many such patients. Genetic muscle diseases are not rare. But acquired muscle diseases such as critical care myopathy, muscle cachexia following cancer, heart failure or renal failure, traumatic muscle ischemia, or myopathy following drug reactions are definitely even more common.
We still have no treatment for any genetic muscle disease. However, we have made marked progress on an understanding of their pathologies. For acquired muscle disease, we are in the process of developing treatment strategies, although as always, “vigilance equals avoidance”.
Team
Head of the group
Simone Spuler, MD, Professor of Myology
simone.spuler@charite.de
simone.spuler@mdc-berlin.de
Phone +49 30 450 540 501
Phone +49 30 450 540 504
Project Officer
Monique Wysterski, M.A.
Member of the Spuler Lab since 2020
monique.wysterski@charite.de
monique.wysterski@mdc-berlin.de
Phone +49 30 450 540 504
Senior Scientist
Andreas Marg, Ph.D.
Member of the Spuler Lab since 2010
Area of expertise: Muscle stem cells
Current project: Satellite cells and heterogeneity
andreas.marg@charite.de
Phone +49 30 450 540 524
Scientists and Physicians
Susanne Behr-Perst, Ph.D.
Member of the Spuler Lab since 2021
Area of expertise: project management for clinical trials, regulatory affairs, audit conduct and CAPA management
Current project: Head of Clinical Trial MuST
susanne.behr-perst@charite.de
Phone +49 30 450 540 576
Helena Escobar Fernandez, Ph.D.
Member of the Spuler Lab since 2016
Area of expertise: Genome editing, Cell transplantation, Muscular dystrophy mouse models
Current project: Gene editing of muscular dystrophy causing mutations in patient cells and novel humanized mouse models
Curriculum Vitae
helena.escobar@charite.de
helena.escobar@mdc-berlin.de
Phone +49 30 450 540 526
Elisabetta Gazzerro, MD, Senior Physician of the Outpatient Clinic
Member of the Spuler Lab since 2017
Area of expertise: Neuromuscular Diseases, Metabolic Diseases
Current project: Immunological impact of muscular dystrophies
elisabetta.gazzerro@charite.de
Phone +49 30 450 540 514
Robin Graf, Ph.D.
Member of the Spuler Lab since 2021
Area of expertise: Bioengineering and Biotechnology
Current project: Genetic safety of gene-edited muscle stem cells
robin.graf@charite.de
robin.graf@mdc-berlin.de
Phone +49 30 450 540 518
Janine Kieshauer, M.Sc. Biochemistry and Molecular Biology
Member of the Spuler Lab since 2017
Area of expertise: Muscle Cell biology, regulatory affairs, Advanced Therapy Medicinal Product (ATMP) development
Current project: Validation of processing and manufacturing human muscle stem cells as an ATMP
janine.kieshauer@charite.de
Phone +49 30 450 540 566
Anne Krause, M.Sc. Molecular Biology
Member of the Spuler Lab since 2018
Area of expertise: Molecular biology, induced pluripotent stem cells (iPSCs)
Curriculum Vitae
anne.krause@charite.de
anne.krause@mdc-berlin.de
Phone +49 30 450 540 518
Ph.D. candidates
Busem Ignak, M.Sc. Molecular Life Science, Ph.D. candidate
Member of the Spuler Lab since 2021
Area of expertise: Molecular and cellular biology, proteomics, human induced pluripotent stem cells, and neuronal differentiation.
Current project: Precise gene editing of LGMD2A causing mutations
busem.ignak@charite.de
Phone +49 30 450 540 518
Supriya Sai Krishna, M.Sc. Molecular and Cellular Biology, Ph.D. candidate
Member of the Spuler Lab since 2022
Area of expertise: Molecular and cellular biology
Current project: Precise gene editing of LGMD2A causing mutations
Curriculum Vitae
supriya.krishna@charite.de
supriya.krishna@mdc-berlin.de
Phone +49 30 450 540 519
Technical Assistants
Stephanie Meyer-Liesener, Head Technician
Member of the Spuler Lab since 2008
Area of expertise: cell culture techniques, lab management
Current project: Regulation und Fehlregulation von Muskelwachstum
stephanie.meyer@charite.de
Phone +49 30 450 540 519
Stefanie Haafke, Biology Laboratory Technician
Member of the Spuler Lab since 2012
Area of expertise: Molecular biology, immunofluorescence staining, cell culture
Current project: Precise gene editing of LGMD2A causing mutations
stefanie.haafke@charite.de
Phone +49 30 450 540 518
Students and Interns
Oskar Johannssen
April - June 2025
A L U M N I
Biniam Bekele, M.D., M.Sc.
Member of the Spuler Lab 2019 - 2022
Former Medical Doctor in Outpatient Clinic for muscle disorders and scientist at MuST-Trial (Clinical Trial, first-in-human)
Current position: Surgical resident at the Department of Cardiothoracic and Vascular Surgery, Deutsches Herzzentrum der Charité – Medical Heart Center of Charité and German Heart Institute Berlin, Germany
Dominique Braumann
Member of the Spuler Lab 2021 - 2022 (parental leave cover)
Former project: Validation of the processes and production of human muscle stem cells as ATMP
Silvia Di Francescantonio, Ph.D.
Member of the Spuler Lab 2017 - 2022
Former project: Bacterial nano-cellulose: human muscle stem cells culture strategy for improving gene-editing approaches
Current position: Postdotoctoral fellow at Edgar Gomes Lab at Instituto de Medicina Molecular João Lobo Antunes, Lisboa, Portugal
Teresa Gerhalter, Ph.D.
Member of the Spuler Lab 2021 - 2022
Former project: suMus - a digital ecosystem around the quantification of muscle activity
Current position: PostDoc at Faculty of Medicine, Friedrich Alexander University, Erlangen, Germany
Henning Langer, Ph.D.
Member of the Spuler Lab 2014 - 2018
Former project: Aminoacid metabolism in muscle wasting
Current position: Post-Doctoral Fellow at Goncalves Lab at Weill Cornell Medicine, Center for Metabolic Health New York, USA
Jakub Malcher, Ph.D.
Postdoctoral fellow 2018 - 2020, MyoGrad PhD fellowship 2013 - 2018
Former project: Exon skipping and genome editing as therapeutic strategies for dysferlinopathy
Current position: Co.Lab Program & Operations Manager at BAYER
Eric Metzler, Ph.D.
Member of the Spuler Lab 2015 - 2022
Former project: Development of a cell therapy concept based on the generation of human induced Pluripotent Stem Cells (hiPSCs) and their differentiation into induced myogenic cells
Current position: Principal Stem Cell Scientist at MyoPax (spin-off)
Stefanie Müthel, Ph.D.
Member of the Spuler Lab 2018 - 2024
Former project: Precise gene editing of LGMD2A causing mutations
Current position: Team Lead R&D Gene-Editing at LONZA
Adrienne Rothe, Biological-Technical Assistant
Member of the Spuler Lab 2013 - 2022
Former project: Human diagnostics, Sgca- mice
Verena Schöwel-Wolf, MD, MBA
Member of the Spuler Lab 2010- 2022
Medical Doctor in Outpatient Clinic for muscle disorders
Former project: Development of preclinical dysferlinopathy model and study of pathomechanism and potential therapeutic strategies, preclinical and clinical study set-up for first-in-human trial MuST
Current position: CEO at MyoPax (spin-off)
Mark Smith, Ph.D.
Member of the Spuler Lab 2021 - 2022
Christian Stadelmann, M.Sc. Translational Medicine, Ph.D.
Member of the Spuler Lab 2020 - 2024
Former project: GMP-compliant Gene Editing in Primary Muscle Stem Cells for Autologous Transplantation
Current: Medical student at the Charité Universitätsmedizin Berlin
Haicui Wang, Ph.D.
Member of the Spuler Lab 2020 - 2022
Former Project: Gene editing in LMNA related muscular dystrophy patient-derived cells
Current position: Group Leader at Department of Human and Animal Cell Lines, the Leibniz Institute DSMZ, Braunschweig, Germany
Former Interns and Students
Magdalena Bolsinger
Gracia Carrero Peralta
Lucia Link Dopazio
Oskar Johannssen
Leon Kersting
Tim Kühnlenz
Marula Mathew
Luise Minkewitz
Yassin Rassafi
Teresa Schätzl
Leon Zitzelbsberger
Research
Gene Editing in Muscular Dystrophies
Helena Escobar Fernandez, Anne Krause, Stefanie Müthel, Christian Stadelmann, Haicui Wang
- Gene editing of muscular dystrophy causing mutations in patient cells and novel humanized mouse models
- Helena Escobar Fernandez
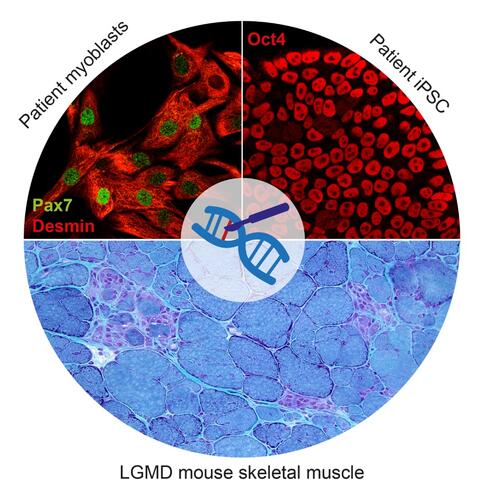
Muscular dystrophies are >40 monogenic diseases characterized by progressive muscle degeneration and atrophy. All of them are currently untreatable. Among them, the term limb-girdle muscular dystrophy (LGMD) comprises about 30 diseases. One of the most severe and frequent types of LGMD is LGMD2D, caused by α-sarcoglycan deficiency due to loss-of-function mutations in SGCA. Another common type of LGMD is LGMD2B, caused by dysferlin deficiency due to loss-of-function mutations in DYSF. My aim is to develop treatments for those diseases based on repairing the genetic defect using CRISPR/Cas9-based methods. I focus on two approaches:
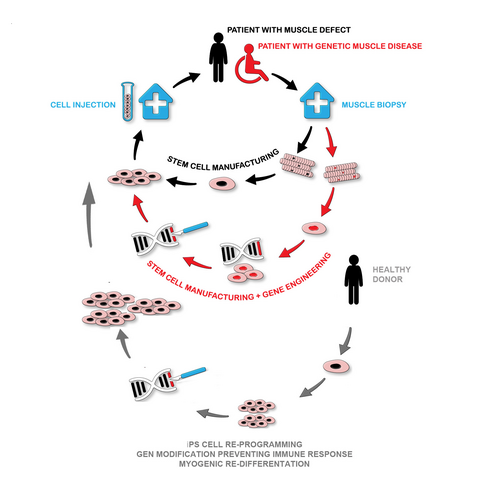
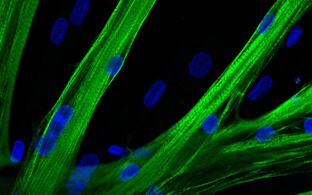
Cell-based gene therapy: Skeletal muscle can regenerate from muscle stem cells and their myogenic precursor cell progeny, myoblasts. We previously shown that human primary myoblasts can be isolated and substantially expanded from muscle biopsies, and that they can regenerate muscle in vivo following intramuscular transplantation into immunocompromised mice (Marg et al., 2014 J Clin Invest; Marg et al., 2019 Nat Commun). A potentially safe and long-term treatment avenue for muscular dystrophy patients is thus cell replacement therapy using autologous gene repaired myoblasts. Another potential autologous source for cell replacement therapies is induced pluripotent stem cells (iPSC). iPSC can be obtained from human adult somatic cells (Takahashi et al., 2006 Cell) and resemble the pluripotent cells in the early embryo. They have unlimited proliferation capacity and can be re-differentiated into almost all adult tissues, including muscle. I focus on repairing muscular dystrophy-causing mutations in SGCA and DYSF in primary myoblasts and iPSC from LGMD 2D and 2B patients, respectively. Our future goal is to develop regenerative therapies for muscular dystrophy patients based on transplantation of autologous gene repaired myogenic cells.
In vivo gene editing: Because skeletal muscle is the most abundant tissue in the body, obtaining sufficient numbers of gene repaired autologous cells to restore function in all affected muscles is a big challenge. An alternative is delivering the gene editing machinery into the body, either via the blood circulation or directly into the muscle. In cooperation with Ralf Kühn (MDC Transgenics Facility), we have generated novel LGMD 2D and 2B mouse models that carry patient mutations in the context of the human SGCA and DYSF gene sequence and recapitulate the patient progressive muscular dystrophy phenotype. I aim to develop in vivo gene editing approaches to repair the mutations in the muscle tissue of those mice, thereby rescuing the phenotype, with the ultimate goal of translating these findings into therapies for LGMD patients.
Other people involved: Silvia Di Francescantonio (repair of DYSF mutations in LGMD2B patient myoblasts and mouse model); Alexej Zhogov and Magdalena Bolsinger (characterization of new LGMD 2D and 2B mouse models)
© Dr. Helena Escobar Fernandez- Precise gene editing of LGMD2A causing mutations
- Stefanie Müthel
Gene editing is a powerful tool to repair disease-causing mutations in patient-derived primary cells. In this project we aim to repair mutations in the CAPN3 gene. Calpain 3, the protein encoded by CAPN3, is a cysteine protease predominantly expressed in the skeletal muscle. Mutations in CAPN3 lead to Limb Girdle Muscular Dystrophy Type 2A, a progressive skeletal muscle disorder without treatment and the most common LGMD worldwide.
In our Outpatient Clinic we have several patients suffering from LGMD2A with different mutations in CAPN3. The project aims to establish an efficient gene editing platform for precise gene correction of disease-causing mutations in primary human muscle stem cells from patients. To assess a successful gene repair, we are also developing methods to determine reliably the rescue of the phenotype in vitro and in vivo. Due to the regenerative potential of primary muscle stem cells, we are confident to use repaired muscle stem cells from LGMD2A patients for an autologous cell therapy treatment.- Gene Edited Primary Human Muscle Stem Cells for Treatments in Muscular Dystrophy
- Christian Stadelmann
Muscle-specific stem cells populate human skeletal muscle and hold great regenerative potential. They can be isolated from patients, expanded, and genetically manipulated in cell culture. These qualities make them a promising candidate for the development of an autologous cell therapy for the treatment of hereditary Muscular Dystrophies.
The heterogeneous group of untreatable muscle wasting disorders is often caused by monogenic mutations that can be targeted with precise CRISPR/Cas-based gene editing tools such as traditional CRISPR/Cas9 editing or the newly developed base editors.
This project aims to develop gene editing strategies that may become suitable for clinical use. This entails optimising and validating the ex-vivo gene editing step to establish a safe and efficient protocol to be translated to the clinic.
- Gene editing in LMNA related muscular dystrophy patient-derived cells
- Haicui Wang
Classical laminopathy refers to diseases caused by mutations in gene LMNA coding for lamin A/C, key components of nuclear lamina on the interior of the nuclear envelope. The majority of classical laminopathy are caused by autosomal dominant LMNA mutations and the clinical phenotypes can vary from muscular dystrophy, dilated cardiomyopathy, Charcot-Marie-Tooth type 2B, to aging phenotype progeria.
We aim to use the state of the art gene editing tools including allele specific CRISPR-Cas9 or CRISPR base editing without double strand breaks to correct the mutations in cells derived from LMNA related muscular dystrophy patients. The screening for the efficient editing tool is carried on with patient derived induced pluripotent stem cells (iPSC). The patient derived muscle stem cells corrected with the validated editing strategy from iPSC are prepared further for the transplantation therapy.
© Dr. Haicui Wang
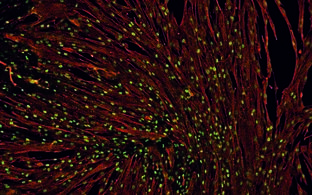
Muscle Stem Cells
Silvia Di Francescantonio, Andreas Marg, Eric Metzler
- Bacterial Nano-Cellulose: Human Muscle Stem Cells Culture Strategy For Improving Gene-Editing Approaches
- Silvia Di Francescantonio
Regenerative capacity of muscle stem cells (satellite cells) makes them the optimal cell type for gene editing and cell-based therapies in the context of monogenic muscle disease. Ideally, they should be isolated from patients, manipulated in vitro, expanded while preserving their stem cell characteristics for autologous transplantation. We aimed to develop a cell culture method that will enable us to perform in vitro manipulations (i.e. gene editing) on human primary myoblasts while avoiding extensive cells propagation, which is known to contribute to exhaustion of stemness. Bacteria nano-cellulose (BNC) substrate was proved to allow myoblasts preservation for many weeks at a very low proliferation rate without evidence of terminal differentiation. Therefore, we considered BNC a well suitable and innovative method for the maintenance of slow-dividing cells in in vitro cultures.
CRISPR/Cas9-based gene editing can be a powerful tool to correct dystrophies-causing mutations (e.g. Limb girdle muscular dystrophy type 2B) however, the repair of slow or non-dividing cells remains a major challenge. BNC was used as a tool for compare gene correction strategies in primary human myoblasts under dividing and slow-dividing conditions.
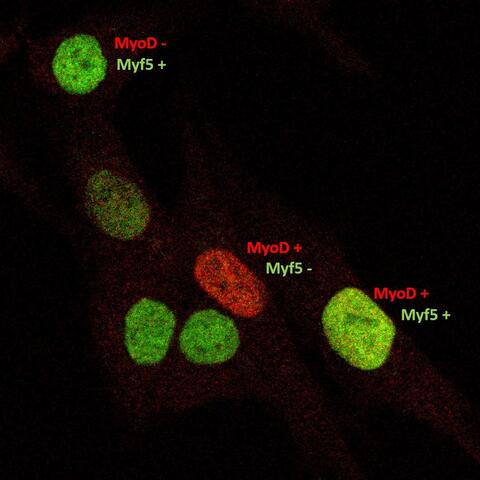
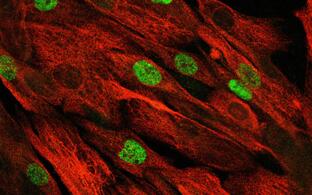
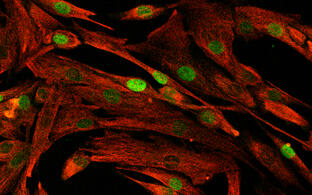
- Satellite cells and heterogeneity
- Andreas Marg
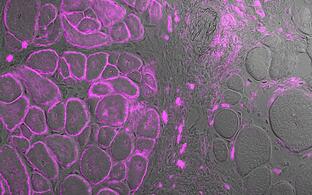
Muscle repair and regeneration require activation of satellite cells. These rare muscle precursor cells are located in a specific niche and are probably mitotically quiescent in healthy muscle. It is unclear to what extent satellite cells are heterogeneous in respect to their gene expression profiles, their myogenic differentiation potential and their stemness. Today, our understanding of human satellite cell heterogeneity is fragmentary, but clinical applications of stem cell populations require extensive knowledge in this field.
In collaboration with the "Berlin Institute for Medical Systems Biology" (BIMSB) we used the drop-seq method for the rapid profiling of single cells. After sequencing, we obtained the mRNA expression profile of thousands of satellite cells. Based on these data, we are now trying to isolate the cells with different cultivation and selection methods that have the best requirements for a successful therapy of muscular dystrophies.
Human activated human satellite cells show heterogeneity.
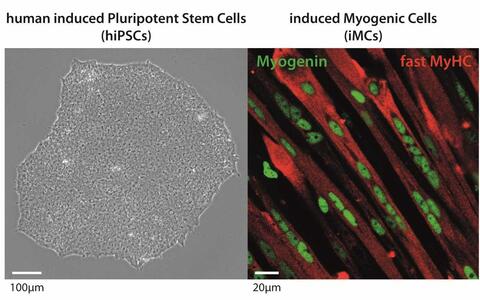
© Dr. Andreas Marg- Development of a cell therapy concept based on the generation of human induced Pluripotent Stem Cells (hiPSCs) and their differentiation into induced myogenic cells
- Eric Metzler
Human induced pluripotent stem cells (hiPSCs) are a keystone to unrestricted cell numbers which are necessary for gene correction and repopulation of large organs such as skeletal muscle in genetic muscular dystrophies. hiPSCs have been generated from many different cell types and several protocols have been established to differentiate them into muscle cells or dedicated muscle stem cells. However, the biotechnological and therapeutic capabilities of these induced myogenic cells remain unclear.
This project aims to develop an efficient in vitro myogenic differentiation strategy, including the comparison of hiPSCs generated from different somatic sources, to finally produce a pure population of induced myogenic cells with a high potential to contribute to myofibre formation in vivo.© Dr. Eric Metzler
Toxic Myopathies
Joanna Schneider
- Epigenetic changes and repair of the DNA breaks in skeletal muscle/or muscle stem cells in critical illness myopathy
- Joanna Schneider
Critical illness myopathy (CIM) is a devastating acquired skeletal muscle disease characterized by atrophy, flaccid paralysis and respiratory failure. It develops in severely ill patients during the course of critical illness and is a frequent complication of intensive care unit (ICU) treatment. It is a very peculiar aspect of CIM that, in some patients, skeletal muscle atrophy and weakness last for a prolonged period of time, often lifelong, although all identified risk factors like inflammation, hyperglycemia, medications etc. have been removed. We hypothesize that the acute phase of severe critical illness leads to epigenetic changes in skeletal muscle stem cells or early myoblasts, which results in an impaired ability of muscles to regenerate, a long lasting myopathy and an increase of DNA double-strand breaks in the muscle cells. Our project aims to identify and characterize the epigenetic modifications in muscle stem cells derived from acute-onset CIM patients within the first days after admission to the ICU. We analyze the epigenome and transcriptome as well as the DNA double-strand breaks process of activated satellite cells and early myoblasts. This project is part of the Clinical Scientist Program of the Berlin Institute of Health and Charité - Universitätsmedizin Berlin.
Application oriented projects
Biniam Bekele, Janine Kieshauer, Verena Schöwel-Wolf
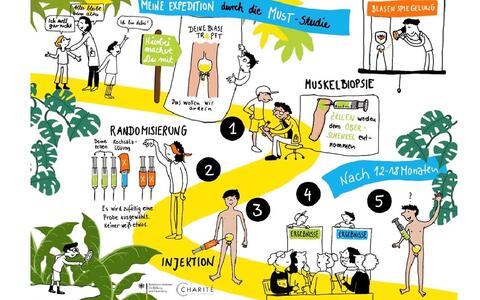
- MuST-Trial: Muscle stem cell therapy for treatment of urinary incontinence in epispadias
- Biniam Bekele
Isolated epispadias is the mildest form of exstrophy-epispadias complex (EEC); a congenital malformation disorder involving the midline abdominal and genitourinary structures. Patients with epispadias have a defect in the urinary sphincter, where muscle tissue is replaced by connective tissue. Thus, these patients suffer from life-long urinary incontinence with a plethora of medical, psychological, social, and financial implications. Currently no causal therapy exists that aims to repair this defect.
Skeletal muscle possesses its own stem cells, the satellite cells. They are highly regenerative cells able to differentiate into myotubes and fuse with existing muscle fibers to form new muscle tissue. Our lab possess several years of experience in manufacturing pure and highly regenerative satellite cell populations (PHSats, Primary human satellite cells).
We are currently preparing this first-in human trial where PHSats will be used to repair the sphincter defect in isolated epispadias patients. We aim to demonstrate the safety and efficacy of our product with achieving urinary continence as our main target. The first patient is scheduled to be included in winter 2021/22.
This trial is financed by the Federal Ministry of Education and Research and supported by the Else-Kröner-Fresenius Foundation. It is a multi-center trial conducted in two of the biggest epispadias treatment centers in Europe; the pediatric urology clinic at the university of Ulm and the Clinic for Pediatric Urology at the University Medical Center Regensburg. We are also in close contact with the patient self-help group Ekstrophie and the clinical and scientific center CURE-Net.
Child-friendly explanation of the study
© AG Spuler- Validation of processing and manufacturing human muscle stem cells as an ATMP
- Janine Kieshauer
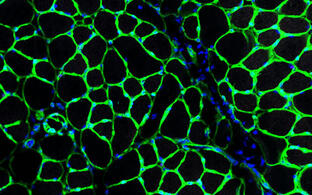
Skeletal muscle, the largest organ of the human body, possesses an own stem cell population, the satellite cells (SCs). Their high regenerative capacity makes SCs a perfect source of cells for cell-based therapies of muscle diseases. We invented a new technology that allows for the first time million-fold expansion of human satellite cells and at the same time the delay of their differentiation. We name our product PHSat (primary human satellite cell product). Hypothermia pretreatment eliminates otherwise co-isolated contaminating fibroblasts. Without the need of a cell-sorting procedure our cell colonies are >98% myogenic (desmin-positive). The muscle tissue is gently mechanically prepared, no enzymatic digestion is performed. By this, we generate native (not activated) and highly regenerative satellite cell populations. The regenerative potential of PHSats has been demonstrated in preclinical efficacy studies: 1. Injected PHSats build muscle fibers, 2. They re-populate the satellite cell niche and 3. They regenerate muscle also after re-injury. The aim of this project is to develop a pharmaceutical manufacturing process to transfer the product PHSat into a clinical study. This process must be carried out under a specific infrastructure, whereby highly standardized parameters must be established in order to characterize the product for regulatory approval in the best possible way. This was enabled by the funding of Pregobio.
The product is currently in a pre-clinical study, where it is being tested for possible harmful effects. These data are essential for the start of the clinical study and the pre-clinical study was funded by Spark.
- Muscle stem cells as ATMP
- Verena Schöwel-Wolf
In the EU, muscle wasting affects over 6 million people and is untreatable. In sarcopenia and genetically caused muscular dystrophies, all muscles are affected. In urinary incontinence or weakness of the diaphragm, only single muscles do not function, yet with dramatic impairment of life quality and life-threatening consequences.
Skeletal muscle possesses its own stem cells, the satellite cells. They promote the high regenerative capacity of skeletal muscle. Our USP is the technology to manufacture highly regenerative satellite cells. Our innovation enables for the first time highly standardized and effective use of these satellite cells in regenerative medicine (PHSats, primary human satellite cells). We aim to develop a satellite cell therapies (-/+ gene engineering) to treat muscle wasting (Figure).
We have analysed the ATMP market and identified pitfalls in ATMP product development. We have developed a strategy for stem cell therapy development in muscle diseases. This strategic product development was awarded in the Science4Life Business Plan Competition 2019.
We are currently preparing a first-in-human phase trial for a first PHSat product. The trial preparation is supported by the Translatorik program of the Else-Kröner-Fresenius Foundation. The aim is to reconstitute a prenatally incompletely developed bladder sphincter muscle and to cure an otherwise lifelong urinary incontinence. We have secured the financing of the trial (Federal Ministry of Education and Research). The first patient is scheduled to be included in the study in winter 2021/22.
In addition, the satellite cell therapy (primary and iPS cell-derived) is combined to gene-engineering strategies and a pipeline is developed to treat a multitude of genetically caused muscular dystrophies.
© Verena Schöwel-Wolf
Clinical Research
- Muscle metabolism in facioscapulohumeral muscular dystrophy
- Investigated Initiated Clinical Trials (IICT): MuST NCT04729582, bASKet NCT05588401
Publications
Patients
Patient organizations (alphabetical order)
- Coalition to Cure Calpain 3
- Overcoming Weakness with Strength, Westport, Connecticut, USA
-
Coalition to Cure Calpain 3 provides support for promising research into finding treatments or a cure for limb-girdle muscular dystrophy, type 2A (LGMD2A/R1, a form of calpainopathy). The unrelenting nature of this disease takes its victims from full mobility to a wheelchair within 11-28 years after the onset of symptoms. LGMD2A/R1 attracts significantly fewer research dollars than other forms of muscular dystrophy and thus fewer researchers working to understand the disease and discover a cure.
The sole focus of C3 is on supporting researchers rather than on providing services to those who have the disease. Thus, all of the funds that are raised go directly into research labs, into bringing researchers together to exchange ideas and collaborate, and into supporting a patient database which would give researchers a list of people to contact about clinical trials of promising therapies.
- DGM
- Deutsche Gesellschaft für Muskelkranke e.V., Freiburg, Germany
-
The Deutsche Gesellschaft für Muskelkranke e.V. (DGM) is the largest self-help organization for people with neuromuscular diseases in Germany. Founded in 1965, it provides comprehensive support for affected individuals, their families, and professionals. The DGM is committed to improving the quality of life for people with muscle diseases by offering counseling, information, and networking opportunities. Additionally, it promotes research and the development of new therapeutic approaches for neuromuscular diseases such as muscular dystrophies, spinal muscular atrophies, and other rare muscle disorders.
- DMH
- Deutsche Muskelschwund-Hilfe e.V., Hamburg, Germany
-
The Deutsche Muskelschwund-Hilfe e.V. (DMH) is a non-profit organization dedicated to supporting people with muscle wasting (neuromuscular diseases). Its mission is to assist affected individuals and their families by providing comprehensive counseling, information, and support services. Additionally, DMH promotes research projects aimed at developing therapies and cures for muscle-wasting diseases, such as muscular dystrophies.
- JAIN FOUNDATION
- LGMD2B | LGMDR2 | Miyoshi, Seattle, Washington, USA
-
The Jain Foundation is singularly focused on finding a cure for dysferlinopathy, also referred to as LGMD2B, LGMDR2, Miyoshi Myopathy 1
- MDA
- Muscular Dystrophy Association Inc., Chicago, Illinois, USA
Since 1950, MDA has been the leading philanthropic organization in advancing understanding and treatment of neuromuscular diseases, driving breakthroughs in genetics, diagnostics, and therapies. Our legacy includes ensuring that people living with neuromuscular disease have access to exceptional care from day one of their diagnosis onward, advocating for inclusion, autonomy, and supporting our community through comprehensive educational resources. Through vibrant community programs, we foster deep connections and empower people living with neuromuscular disease to experience the world in the way that they want to, turning challenges into triumphs. Read more about MDA's journey and the progress we've helped make possible. https://www.mda.org/
- SFCM
- Strong for cured muscles e.V., Westerstetten, Germany
-
Strong for Cured Muscles e.V. is a non-profit organization based in Westerstetten, Germany. The organization is committed to raising awareness about rare muscle diseases and supporting research in this field. Its goal is to promote therapeutic approaches that could provide long-term cures for various muscular disorders. Additionally, the organization is actively involved in public outreach and supports affected individuals and their families through fundraising campaigns and other initiatives aimed at increasing awareness of neuromuscular diseases.