Daumke Lab
Structural Biology of Membrane-Associated Processes
Profile
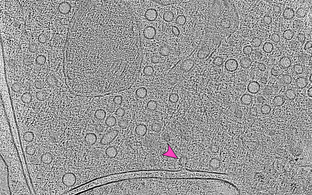
Artistic view on the pinching of clathrin-coated vesicles by the mechano-chemical GTPase dynamin. Structural work in our group has clarified the oligomerization mode of dynamin on membranes and led to a GTPase-driven ratcheting model leading to membrane constriction.
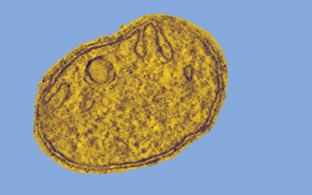
The shape of cellular membrane is intimately linked to cellular function. We explore structure, function and mechanism of molecular machines that remodel cellular membranes to facilitate membrane trafficking and the maintenance of cellular organelles. To this end, we apply a combination of structural biological, biochemical and cell biological methods.
Dysfunction of such molecular machines, for example by mutations, is often associated with human disease, such as muscular dystrophy or neurodegenerative disorders. Our research contributes to a molecular understanding of the disease phenotypes and may contribute to the development of new therapeutic approaches.
See ‘Research’ for specific project descriptions.
Team
Research
A short overview on our recent projects is shown below.
Publications
News
Teaching material
You find diverse teaching material (in German) from us and other groups of the Collaborative Research Grant 958 here: