M-line deficient titin causes cardiac lethality through impaired maturation of the sarcomere
Titin is a giant muscle protein that forms a continuous filament system in both skeletal and cardiac muscle. It is composed of different functional units that enable titin to integrate into the Z-disc and M-band, provide elasticity, and support sarcomere assembly. The presence of phosphorylation sites and a kinase domain suggest a role for titin in signal transduction and also for regulating sarcomere assembly via its substrate titin cap (T-cap)
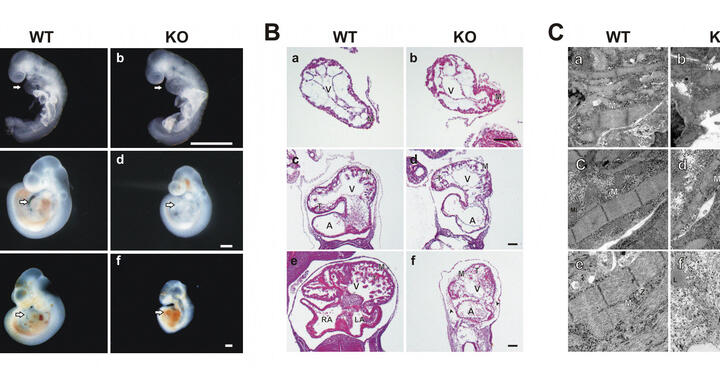
In a new study, Stefanie Weinert (laboratory of Prof. Michael Gotthardt) and colleagues set out to elucidate the function of titin’s kinase region in myofibrillogenesis. The researchers generated an M-line deficient titin knockout. Homozygous deficient mice develop normally until embryonic day 9. From E9.5 cardiac growth is delayed, followed by disassembly of the sarcomere, inability of the heart to contract, and lethality by E11.5. Immunofluorescence analysis revealed periodic staining of sarcomeric proteins such as α-actinin and the heart specific titin-N2B region, indicating correct I-band assembly in nascent myofibrils. Unlike in the adult knockout, kinase deficient titin did not integrate into the M-band and, thus, failed to form a continuous filament. Ultimately, this structural deficiency lead to sarcomere disassembly with increased mechanical strain.
The findings suggest diverse functions for titin in embryonic development and in the adult heart, which does not only involve differential expression of titin isoforms, but also of titin binding proteins. The switch between the fetal and adult expression pattern could be an attractive therapeutic target to balance the stability of the sarcomere and the efficiency of contraction.
Contact:
Pamela Cohen
p.cohen@mdc-berlin.de
+49 30 9406 2121