Daumke Lab
Strukturbiologie Membran-assoziierter Prozesse
Molecular basis of protein acetylation
N-terminal acetylation is a co-translational modification known to modify protein stability and interaction. Lysine acetylation regulates protein-protein and protein-DNA interactions. Importantly, both modifications have been implied in regulating the function of peripheral membrane proteins. While N-terminal acetylation of the small GTPase ArfRP1 by the NatC complex has been shown to regulate its Golgi recruitment, lysine acetylations may affect protein-membrane interactions.
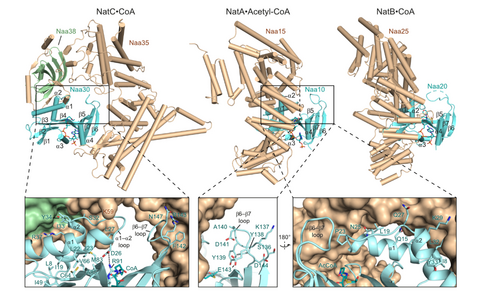
Figure 1: Structure of the heterotrimeric NatC complex (pdb 6YGA), which shows a different architecture compared to previously determined NatA and NatB complexes (pdb 4KVO and 5K04, respectively).
To characterize the molecular basis for these activities, we determined the crystal structure of the yeast heterotrimeric NatC complex, which exhibits a strikingly different architecture compared to previously described N-terminal acetyltransferases (NATs) (Grunwald et al., Nat Commun, 2020). Cofactor and ligand-bound structures revealed the molecular basis of how the first four amino acids of cognate substrates are recognized at an interface between two subunits. A sequence-specific, ligand-induced conformational change enables efficient acetylation. Based on detailed structure-function studies, we suggested a catalytic mechanism and identified a ribosome-binding patch in an elongated tip region of NatC. Our study reveals how NATs have divergently evolved to N-terminally acetylate specific subsets of target proteins.
In a collaborative study with the group of Ralf Langen/USC, we characterized the function of lysine acetylation in dynamin-like EHD2 and found reduced membrane-binding and remodeling in an acetylated EHD2 variant (Okada et al., Nat Commun, 2021). In a systematic fashion, the Langen group then characterized lysine acetylations in a range of membrane-remodeling proteins. These data suggested that lysine acetylation is a widespread mechanism for regulating membrane interactions in peripheral membrane-binding proteins.
Publications
- Grunwald, S., Hopf, L.V.M., Bock-Bierbaum, T., Lally, C.C.M., Spahn, C.M.T., and Daumke, O. (2020). Divergent architecture of the heterotrimeric NatC complex explains N-terminal acetylation of cognate substrates. Nat Commun 11, 5506. https://doi.org/10.1038/s41467-020-19321-8.
- Okada, A.K., Teranishi, K., Ambroso, M.R., Isas, J.M., Vazquez-Sarandeses, E., Lee, J.Y., Melo, A.A., Pandey, P., Merken, D., Berndt, L., et al. (2021). Lysine acetylation regulates the interaction between proteins and membranes. Nat Commun 12, 6466. https://doi.org/10.1038/s41467-021-26657-2.
Researchers in my group
Elena Vazquez Sarandeses (Doctoral student)
Stephan Grunwald (Doctoral student 2014 – 2019)
Main collaborations
Ralf Langen, University of Southern California.